Scientific Foundations Primer
Download PDFWater
Water is essential to life, and approximately 50% to 60% of our bodies are composed by weight of water. The water in our bodies is distributed into three compartments: blood, intracellular fluid (cytoplasm) and fluid surrounding cells (interstitial). All molecules and chemicals in our bodies must interact with water to become soluble to generate the structures and perform the reactions that sustain life. Understanding the basic properties of water and how molecules interact with and move in water is essential for understanding how cells, tissues and organs function to support life.
Properties
The structure of water is simple: a single oxygen atom forming single covalent bonds to two hydrogen atoms. But that simple structure and the chemical features of oxygen and hydrogen create unique properties of water that are critical for life.

The most important property of a water molecule is its charge polarity. Even though a water molecule does not contain an overall net charge, charge is unevenly distributed across a water molecule which creates polarity. The oxygen atom in water more strongly attracts electrons in the covalent bonds which creates a slight negative charge near the oxygen atom and a slight positive charge near hydrogen atoms. In addition, oxygen-hydrogen covalent bonds are bent, forming an angle of approximately 104˚. If water were a linear molecule (bond angle of 180˚), then the charge distribution along the bonds would cancel out. The bend creates a negative charge near the oxygen atom and a positive charge located approximately between the hydrogen atoms. This is called a dipole moment and underlies many of the macroscopic properties of water that are familiar to us in our daily lives and essential for supporting life.
One critical property of water is that it remains in liquid form at relatively high temperatures (100˚ C/212˚ F). Water’s ability to remain a liquid is due to the interaction between individual water molecules. The dipole moment in water allows the positive end of one water molecule (hydrogen) to interact with the negative end of another (oxygen). This type of bond is called a hydrogen bond is fundamental to the interactions between biological molecules. Because each water contains two hydrogen atoms, large chains of interacting water molecules can form. The network of interactions between water molecules means more energy (heat or higher temperature) is required to convert water from a liquid to gas.

Behavior of Biological Materials in Water
The polarity of water is also critical for its properties as a solvent. Water’s polarity allows it to interact with and dissolve molecules and chemicals with electrical charges. Importantly, most salts readily dissolve in water (e.g. NaCl, KCl). The polarity of water allows it to break the ionic bond between sodium and chloride to generate a positively charged sodium ion and negatively charged chloride ion. The water in our bodies and in our cells have specific amounts of certain ions, and maintaining the concentration of those ions is critical for the survival and function of every cell.

Many other important biological molecules readily dissolve in water due to the charge polarity on their surface and ability to form hydrogen bonds with water. These types of molecules are called hydrophilic.
In contrast, molecules or portions of molecules that lack polarity do not interact with water and are called hydrophobic. Because they do not interact with water, hydrophobic molecules interact with each other to avoid interacting with water. Some large molecules, such as proteins, contain both hydrophobic and hydrophilic regions. The hydrophobic regions in a protein often cluster in the interior of the molecule to avoid interacting with water and help the protein fold into a three-dimensional structure. The hydrophilic regions usually reside on the surface of the molecule where they interact with water and solubility the protein.
Diffusion
Recall that one of the properties of water is that it remains in liquid form over a large range of temperatures and at relatively high temperatures. The high temperature of water in our bodies is a result of the thermal motion of the water molecules. The random movement of water molecules imparts movement of the solutes in water. Because the movement of water molecules is random, solutes in water move randomly, too.
Solutes distribute randomly in a fluid. For example, if drop of a concentrated solute is added to a large volume of water, eventually that solute will become evenly distributed throughout the water. Thus, we say the all ions and molecules show a net movement from regions of high concentration to regions of low concentration or that solutes move down their concentration gradient. The rate of diffusion is determined in part by the size of the concentration gradient: a larger gradient generates faster rates of diffusion. Other factors that control the rate of diffusion of a solute include temperature and the size and shape of the solute.
In physiological systems, we are often interested in the movement of solutes between two fluid compartments (e.g, between cytoplasm and extracellular fluid). As a macroscopic example of this movement, let’s place equal volumes of two solutions of sodium chloride in separate chambers. The solution in the left chamber contains 1 M NaCl and the one in the right chamber contains 0.1 M NaCl. The chambers are separated by a membrane that is permeable to sodium and chloride ions. Thus, sodium and chloride ions can freely diffuse from one chamber to the other across the membrane. What will happen to the concentration of sodium and chloride in each chamber over time?
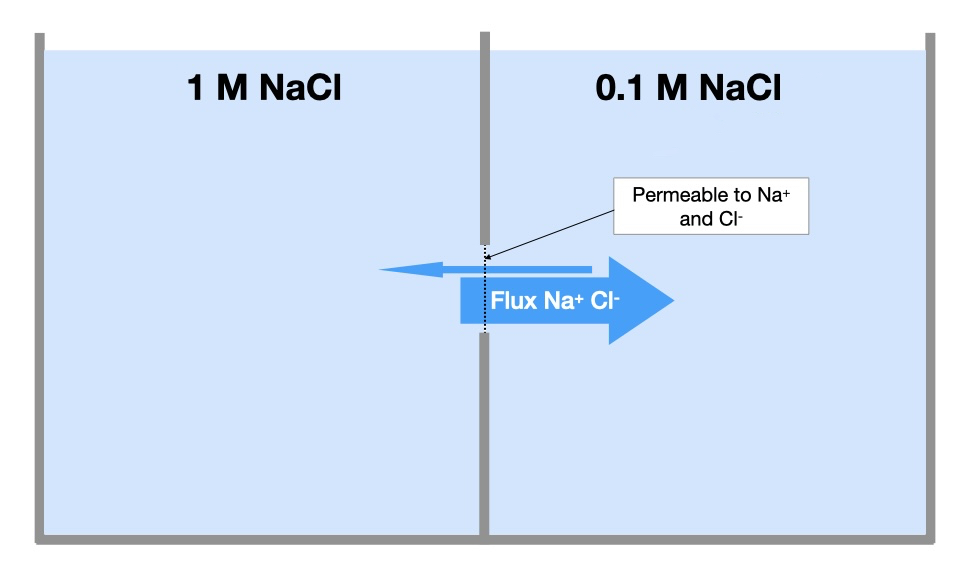
Diffusion dictates that solutes move down their concentration gradients, which means sodium and chloride will move from the left chamber to the right chamber. This will cause the concentration of sodium and chloride ions in the left chamber to decrease and the concentration in the right chamber to increase. The rate of diffusion in this case is also determined by the size of the membrane and its permeability to the ions, in addition to the factors listed above.
When will the movement of sodium and chloride ions between the two chambers end? As long as there is thermal energy in the solutions, the ions will constantly move through the pores from one chamber to the other. However, as the sodium and chloride concentrations continue to decrease in the left chamber and increase in the right, the system will reach a state in which both chambers contain equal concentrations of sodium and chloride. The concentrations of sodium and chloride in each chamber will stop changing because there is an equal probability of ions moving in both directions across the membrane. At this point, the two chambers are in equilibrium with regard to sodium and chloride ions. Even though both ions are still moving between chambers, the number of ions moving from the left chamber to the right is approximately equal to the number of ions moving from the right chamber to the left. Thus, the ions can move between chambers without causing a net change in their concentration in each chamber.

Equilibrium
Equilibrium is an example of steady state. Steady state occurs when the variables of a system do not change over time. In our example, the system consists of the two chambers of NaCl in water and the variables are the concentrations of sodium and chloride in each chamber. Equilibrium satisfies this definition of steady state because the concentration of sodium and chloride do not change over time even though ions are moving across the membrane. The flow of ions in both directions is equal which keeps the concentrations in the chambers the same
The chamber could also be in steady state without being in equilibrium. For example, we could attach to the right chamber a small filter that selectively absorbs sodium and chloride ions from solution. Any ion that collides with the filter would be removed from solution, causing the concentration of sodium and chloride in the right chamber to decrease. We also attach a different type of filter to the left chamber that releases sodium and chloride ions into solution. The filters are designed so that they release or absorb ions at the same rate. Thus, ions enter the left chamber at the same rate as they leave the right chamber.

This system will create a steady state condition that is not in equilibrium. Because ions are added to the left chamber and removed from the right chamber, the left chamber will have a higher concentration of ions than the right chamber. Similar to the example above, the concentration gradient will favor the flow of ions from the left chamber into the right. Despite this directional flow of ions, the concentration of ions in both chambers will not change because ions flowing out of the left chamber are replaced by ions released from the filter, and the filter in the right chamber absorbs an equal number of ions that enter from the left chamber. This net flow of ions from left to right means the system is not in equilibrium, but the system is still in steady state because the concentration of ions in the chambers does not change.
Most reactions in biochemical pathways in our cells are in non-equilibrium steady state. For a given biochemical step in a pathway, the reactants for that step are constantly produced by the upstream reaction and the those products of that step are consumed by downstream reactions.
How Solute Charge Affects Diffusion
The net charge of solute can affect its diffusion, especially when moving across semi-permeable membranes. Recall that when many chemicals (e.g. salts) dissolve in water, they generate an ion or molecule with a net positive or negative charge. The diffusion of ions and molecules with a net charge are affected by surrounding electrical fields. Electric fields impart a force on charged ions and molecules. Positively charged ions and molecules are pulled toward the negative end of an electric field and pushed away from the positive end, whereas negatively charged ions are pulled toward the positive end of the field and pushed away from the negative end.
To illustrate the effect of an electric field on the diffusion of ions, let’s set up our two chambers as before with one containing 1.0 M NaCl and the other containing 0.1 M NaCl and mannitol, but this time will use a membrane that is only permeable to sodium ion. Thus, sodium ions can freely diffuse across the membrane but chloride ions cannot.
First, let’s consider how the sodium and chloride ions are distributed in each chamber. As described above, diffusion randomly distributes solutes in a fluid, but does the charge of the ions affect their distribution? The electric field generated by sodium ions will repel other sodium ions but attract chloride ions. The opposite is the case for chloride ions. Because the number of sodium ions is equal to the number of chloride ions in each chamber, the push and pull effect of same and opposite charges will lead to a random distribution of ions throughout the fluid.

Because the membrane is permeable only to sodium ions, they will diffuse down their concentration gradient and more sodium ions will move from the left chamber to the right than move in the opposite direction. As sodium ions enter the right chamber, the number of sodium ion becomes greater than the number of chloride ions.
How are these excess sodium ions distributed in the right chamber? Because the chamber does not contain enough negative charges to balance the excess positive charges, the excess positive charges (sodium ions) get pushed to the periphery of chamber. Something similar is happening the left chamber. As sodium ions leave the left chamber, chloride ions begin to exceed sodium ions. These chloride ions are also pushed to the periphery of the left chamber.
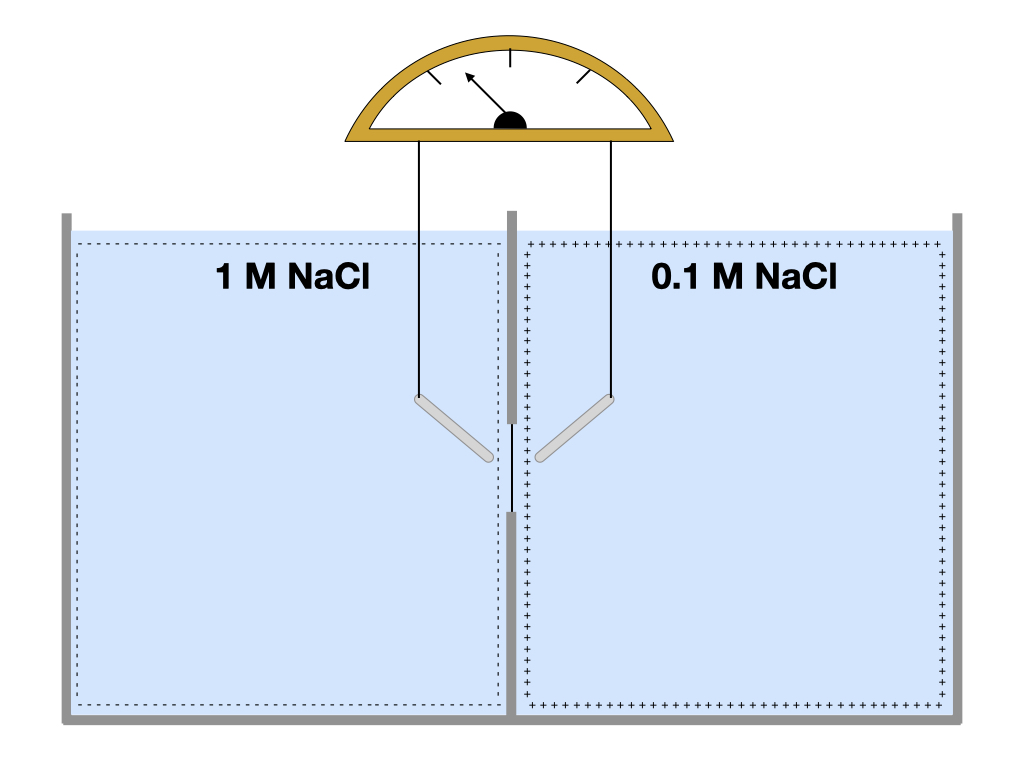
Pay close attention to the membrane through which sodium ions pass between chambers. The right side of the membrane has more sodium ions, generating a positively charged electric field on the right side. The left side of the membrane has more chloride ions, generating a negatively charged electric field on the left side. If we were to place electrodes on either side of membrane, we would measure a voltage across the membrane.
The voltage across the membrane impacts the diffusion of sodium ions. The positive electrical field on the right side of the membrane repels the diffusion of sodium ions from the left chamber into the right, and the negative electric field on the left side of the membrane will attract sodium ions into the left chamber. As more sodium ions diffuse into the right chamber, more sodium ions accumulate on the right side of the membrane and more chloride ions accumulate on the left side of the membrane, strengthening the electric field (measured as voltage). Eventually, the voltage reaches a level where sodium ions are inhibited from diffusing across the membrane.
The amount of voltage across the membrane needed to prevent diffusion of an ion depends on the ratio of concentrations of the ion in the two chambers. A larger ratio requires a large voltage across the membrane. As you will learn in the physiology thread the movement of ions between two fluid compartments (e.g., inside and outside a cell) depends on their concentrations in the two compartments and the voltage across the cell membrane.
Osmosis
Osmosis describes the net movement of water between two compartments due to an unequal number of solutes each compartment. If two compartment are separated by a membrane that is permeable to water but not the dissolved particles, water will move from the compartment with fewer dissolved particles to the one with more dissolved particles (in our example from the right chamber to the left). Consequently, the volume of fluid in left chamber will increase. We can measure the osmotic pressure generated by osmosis by placing a piston on top of the left chamber and applying pressure to the fluid in the chamber. The amount of pressure that prevents the volume in the left chamber from increasing is a measure of the osmotic pressure.

To determine whether water will flow between two compartments, we need to determine the osmolality of the solutions in each compartment. Osmolality is the number of moles of osmotically active particles per kg of solvent which in our case is water. Hence, 1 osmole is 1 mole of an osmotically active particle in a solution that contains 1 kg of water. Because it is easier to measure the volume of a solution rather than the weight of its solvent, some solutions are expressed in osmolarity which is moles of osmotically active particles per liter of solution. Which one should you use? For most fluids found in human body their osmolality will be roughly equally to their osmolarity so either measurement is acceptable. However, certain tests return results in either osmolality or osmolarity so it’s important to be familiar with both.
Osmosis and Cell Volume
Osmosis impacts a cell’s volume and potentially leads to damage and cell death. The cytoplasm of cells contains many proteins, carbohydrates, amino acids, sugars and other small molecules. Each of these counts as an osmole. Thus, if placed in pure water, the number of solutes would be higher inside the cell than outside and water would enter the cell from the surrounding fluid. The influx of water would cause cells to rapidly swell and potentially burst.
Cells employ different mechanisms to prevent bursting. Many single cell organisms, such as bacteria and yeast, have an external cytoskeleton called a cell wall that is composed of protein and carbohydrates. The cell wall physically prevents the cell from swelling if it is placed in a hypoosmotic solution such as pure water. The cells in multicellular organisms don’t have cell walls so need a mechanism to maintain an osmotic balance with their surrounding solution. The course will describe how cells maintain a proper balance of osmoles between their cytoplasm and extracellular fluid.
Acids and Bases
Of particular biological and medical importance is the concentration of hydrogen ion (H+) in the body fluids. The concentration of H+ in a fluid is called its pH and is measured using a log scale:
pH = -log10[H+]
A higher concentration of H+ generates a lower pH value.
Pure water contains a small concentration H+ and OH- because a small fraction of H2O dissociates.. At 25˚ C, pure water has 1 x 10-7 M H+ and 1 x 10-7 M OH-. Because the concentration of H+ equals the concentration of OH-, pure water is considered neutral. Compounds that increase the concentration of H+ when added to water are called acids, whereas those that decrease the H+ concentration are called bases.
The strength of an acid or base depends on its ability to lose (acid) or gain (base) a hydrogen ion. When dissolved in water, a strong acid will almost completely ionize so that almost all of the acid has released a hydrogen ion. In contrast, in weak acids only a small fraction of the acid ionizes. For example, let’s consider an acid HA that dissociates into H+ and A- in water. The strength of the acid can be described by this formula:
Ka = [H+] x [A-]/[HA]
A strong acid has a large Ka value because more of the acid as dissociated into H+ and A -. A weak acid has a smaller Ka.
The A- in our solution is called the conjugate base. If the acid has a large Ka (readily dislocates), the conjugate base (A-) is considered a weak base. If the acid has a small Ka, it is considered a weak acid and its conjugate base is a strong base.
In medicine, we are often concerned with the extent to which a molecule ionizes given the pH of the surrounding fluid. Although most fluids in the body range from pH 7 - 7.4, some fluids, such as the fluid in the lumen of the stomach can be very acidic. To determine the fraction of molecule that is ionized we can use the Henderson-Hasselbach equation which for acids is
pH = pKa + log10([A-]/[HA])
where HA is the concentration of unionized molecule and A- is the concentration of ionized molecule. pKa is the negative log of Ka (-log(Ka)). We need to convert Ka into its negative log because pH is the negative log of hydrogen ion concentration. A strong acid with a large Ka will have a low pKa, and a weak acid with a small Ka will have a large pKa.
Let’s look at a molecule that is a weak acid with a pKa of 6. If the plasma pH is 7.4, then the ratio of ionized to unionized molecule is
7.4 = 6.0 + log10([A-]/[HA])
1.4 = log10([A-]/[HA])
25 = [A-]/[HA]
So, the concentration of ionized molecule is 25-fold more than the concentration of nonionized. What if the molecule were ingested and reached the stomach? In this case with the pH of the stomach about 2.0, the ratio of ionized to unionized molecule changes dramatically:
2.0 = 6.0 + log10([A-]/[HA])
-4.0 = log10([A-]/[HA])
0.0001 = [A-]/[HA]
In the stomach, the concentration of unionized molecule is 10000-fold higher than the amount of ionized.
Knowing whether a molecules is ionized or unionized has important biological and medical consequences. Because an ionized molecule has a charge, it reduces its ability to diffuse across cell membranes, whereas unionized molecules that lack an overall charge can more easily diffuse across cell membranes. This will be important when we consider to what extent drugs are absorbed into the body.
Electrons
You may have thought you saw the last of these tiny, charged particles in your final chemistry or physics class, but the transfer of electrons from one molecule to another plays a critical role in biology and in particular, in many metabolic reactions. Perhaps, the most well-known metabolic reaction is the complete breakdown of glucose into carbon dioxide and water:
C6H12O6 + 6 O2 -> 6 H20 + 6 CO2
Cells use the energy released by the breakdown of glucose to generate ATP from ADP.
C6H12O6 + 6 O2 + 36 ADP -> 6 H2O + 6 CO2 + 36 ATP
Hidden beneath this reaction of molecules is the transfer of electrons from glucose to oxygen which is responsible for generating 34 of the 36 molecules of ATP. Breaking down the reaction into two half reactions reveals the movement of electrons:
C6H12O6 + 6 O2 -> 6 CO2 + 24 e- + 24 H+
6 O2 + 24 e- + 24 H+ -> 12 H20
These two reactions are an example of an oxidation-reduction pair. The top reaction is the oxidation of glucose which results in the loss of 24 e- from the 6 carbons in glucose. In the reduction reaction, those 24 e- are accepted by oxygen to form water.
How do the electrons get from glucose to oxygen? The transfer of electrons is indirect and proceeds through intermediaries and some these intermediaries will help the cell convert ADP to ATP. Most important for the breakdown of glucose are two sets of metabolites: NAD+/NADH and FAD/FADH2
NAD+ + 2 e- + H+ -> NADH
FAD + 2 e- + 2 H+ -> FADH2
Cells will transfer the electrons in NADH and FADH2 to proteins that compose the electron transport chain. These proteins will use energy of electron transfer to generate a proton gradient that will power the generation of ATP from ADP.
Because the transfer of electrons between molecules happens often in biological reactions (and inorganic reactions), the reactions have specific names. A reaction in which a molecule (or atom) loses an electron is called oxidation, and a reaction in which a molecule (or atom) gains an electron is called reduction.
Oxidation: A -> 1 e- + A+
Reduction: B+ + 1 e- -> B
Keep in mind that although the reaction in which an electron is lost is called oxidation, the reaction does not always involve oxygen (though in many biological reactions oxygen is involved).
Oxidation and reduction reactions are usually coupled so that the electron lost in the oxidation reaction is consumed by the reduction reaction. The pairing of reactions leads to some confusing nomenclature. A molecule that is oxidized (A in the above reactions) is said to be a reducing agent because it facilitates the reduction of another molecule (B in the above reactions). Likewise, B is termed an oxidizing reagent because it facilitates the oxidation of A.
As you’ll learn, many metabolic reactions involve the transfer of electrons to or from a biological molecule (e.g. glucose, lipid) using metabolite pairs such as NAD+/NADH, FAD/FADH2, NADP+/NADPH. In each of these pairs, the first molecule is the oxidizing reagent because it accepts electrons which helps oxidize another molecule. The second molecule in each pari is the reducing agent. Cells maintain specific ratios of oxidizing to reducing agents to help drive biological reactions.
Reactive oxygen species
One consequence of the transfer of electrons onto oxygen is the generation of reactive oxygen species:
O2 + e- -> O2-
As the name suggests, O2- and similar reactive oxygen species are highly reactive and can chemically modify many essential biochemical macromolecules (protein, lipid, nucleic acids). These chemical changes modify the structure and activity of the macromolecules and usually shorten their half-life. As a result, the functions and health of cells is reduced which can put an organism into a disease state. As we’ll discuss in the course, cells have several countermeasures to eliminate reactive oxygen species, but over time, all macromolecules incur some damage and require replacements.