Structure and Function of Muscle and Nervous Tissue
Skeletal Muscle
Muscles are multicellular contractile units. They are divided into three types:
- Skeletal muscle
- Cardiac muscle
- Smooth muscle
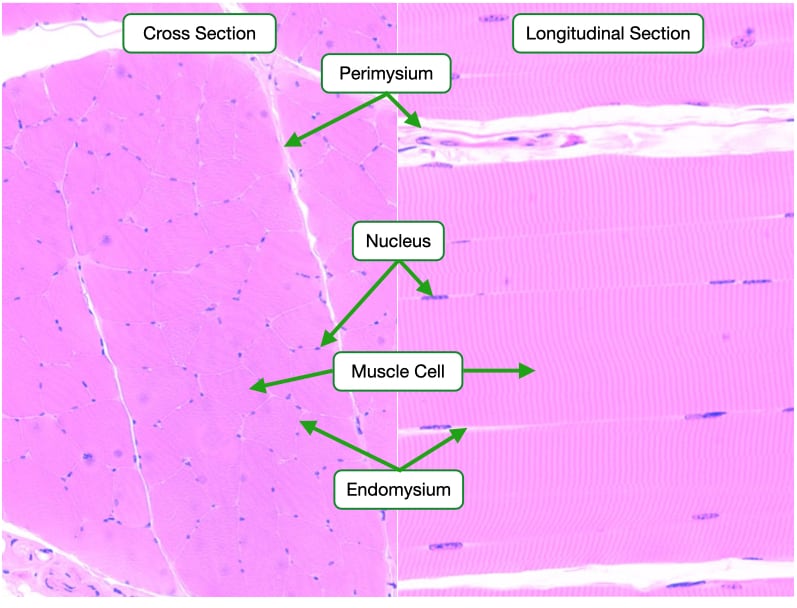
Skeletal muscle is made up of elongated cylindrical multinucleate cells, also called muscle fibers, that span the length of the muscle. Each muscle cell is surrounded by connective tissue called endomysium. Endomysium is similar in composition to basement membrane that underlies epithelia. Muscle cells are grouped together into fasicles and surrounded by a connective tissue called perimysium that is rich in collagen fibers. Finally, several fasicles are bundled to form the whole muscle. The whole muscle is surrounded by a connective tissue layer called the epimysium. Blood vessels and nerves travel through the epimysium, perimysium and endomysium to reach the individual muscle fibers.

Organization of Skeletal Muscle
Skeletal muscle cells are easily identifiable in histological samples. In cross section, skeletal muscle cells appear as large cells with their nuclei located to one side. In longitudinal section, the striations identify skeletal muscle cells. The cytoplasm of skeletal muscle cells is organized into long cables called myofibrils that span the length of the cell. The cells are divided longitudinally into numerous sarcomeres that give skeletal muscle cells their striated appearance.
At high magnifications, the endomysium is more apparent around each muscle cell. Note the presence of capillaries in the endomysium. The perimysium is thicker and contains larger blood vessels (arterioles) that distribute blood to the capillaries in the endomysium.

Sarcomere Electron Micrograph
Sarcomeres are the contractile units in both skeletal and cardiac muscle cells. Sarcomeres are defined by Z-discs and composed of actin filaments and myosin filaments. Z-disc bind the plus ends of actin filaments which extend toward the center of the sarcomere and end at the H-band. The H-band does not contain actin filaments in a relaxed sarcomere. Myosin filaments sit in the center of the sarcomere and span the length of the A-band. A-bands are darker in electron micrographs and H&E-stained samples because they contain both actin filaments and myosin filaments. I-bands are lighter because they only contain actin filaments. During contraction, the myosin motors pull on the actin filaments which bring the Z-disc closer to shorten the sarcomere and the overall muscle cell.
Also note the T-tubule which is an invagination of the cell plasma and allows action potentials that start on the surface of the muscle cell to penetrate into the center of the cell. The sarcoplasmic reticulum runs adjacent to the T-tubule. The sarcoplasmic reticulum is the endoplasmic reticulum of skeletal muscle cells. Recall that action potentials in the T-tubule trigger the opening of calcium channels in the sarcoplasmic reticulum which increases the concentration of cytosolic calcium and triggers muscle contraction.

Types of Skeletal Muscle Cells
Most muscles contain a mixture of these extreme fiber types. In humans, the fiber types cannot be distinguished based on gross examination, but require specific stains or treatments to differentiate the fibers.
Skeletal muscles are divided into two muscle fiber types. Slow-twitch (type I) muscle fibers contract more slowly and rely on aerobic metabolism. They contain large amounts of mitochondria and myoglobin, an oxygen-storage molecule. These muscles can maintain continuous contraction and are useful in activities such as the maintenance of posture.

Fast-twitch (type II) muscle fibers contract more rapidly due to the presence of a faster myosin. Type II fibers can be subdivided into those that have large amounts of mitochondria and myoglobin and those that have few mitochondria and little myoglobin. The former primarily utilize aerobic respiration to generate energy, whereas the latter rely on glycolysis. These muscles are important for intense but sporadic contractions; for example, those that take place in the biceps.
Neuromuscular Junction
Muscle cells innervated by motor neurons that arise from spinal cord. Activation of motor neuron triggers contraction of muscle cell. The axon of motor neurons form synapse on the surface of skeletal muscle cells at the neuromuscular junction.

Neuromuscular junctions are rarely seen in histological samples and require special preparation of muscle tissue. This image shows a single axon from a motor neuron splitting to form synapses on multiple skeletal muscle cells. An axon and the muscle cells it innervates form a motor unit. Some motor neurons innervate one or a few muscle cells whereas other motor neurons can innervate hundreds of muscle cells. Motor axons terminate in a neuromuscular junction on the surface of skeletal muscle cells. The neuromuscular junction occurs at the center of the muscle cell so that action potentials being in the middle of the cell and propagate towards the end of the muscle cell.

Neuromuscular Junction Synapse
The neuromuscular junction is composed of a pre-synaptic axon terminus and a post-synaptic muscle cell. Upon depolarization of the axon, synaptic vesicles containing the neurotransmitter acetylcholine fuse with the membrane, releasing acetylcholine into the cleft that separates the axon from the skeletal muscle cell. Acetylcholine binds to receptors on the post-synaptic membrane. The acetylcholine receptor is a ligand-gated ion channel that opens after binding acetylcholine. The open channel depolarizes the membrane which can trigger opening of voltage-gated sodium channels. The opening of the sodium channels initiates an action potential that propagates along the cell membrane and into the T-tubules.

Note the basal lamina that surrounds the muscle cell. The basal lamina is similar to the basement membrane of epithelia. The basal lamina of skeletal muscle cells contains acetylcholinesterase which is an enzyme that digests acetylcholine. Acetylcholinesterase helps limit the duration of each contractile event.
Cardiac Muscle
Cardiac muscle is the tissue in the heart. In contrast to skeletal muscle where a single cell spans the entire length of the muscle, cardiac muscle consists of many smaller cells, called a cardiomyocytes, arranged in series. Individual cells are linked and communicate via gap junctions which allows action potentials to pass from one cell to the next. Note that the cells are arranged in parallel arrays to generate contraction in one direction.
Similar to skeletal muscle cells, cardiomyocytes have actin and myosin filaments arranged in sarcomeres and each cardiomyocyte is wrapped in a layer of connective tissue.

Cardiomyocytes are much shorter than skeletal muscle cells and are arranged in series to span the length of the muscle. Cardiomyocytes also branch at their ends to form connections with multiple adjacent cells, resulting in a complex, three-dimensional network.
Cardiomyocytes contain one to two nuclei located centrally within the cells. The cells contain an abundance of mitochondria to meet the energy demands of the cells and are surrounded by numerous capillaries.
Cardiomyocytes are joined end to end by specialized junctional regions called the intercalated discs. The intercalated discs allow for the spread of action potential between cells and the transmission of force throughout the muscle tissue.

Cardiac Muscle Electron Micrograph
Similar to the cytoplasm of skeletal muscle cells, the cytoplasm of cardiac muscle cells is organized into sarcomeres. Note the presence of Z-discs and alternating I and A bands. Also note the numerous mitochondria. The dark line is an intercalated disc that connects two adjacent cardiac muscle cells. The intercalated discs contain three types of membrane-to-membrane contacts:
- Fascia adherins use cadherins which are linked to actin filaments via alpha and beta catenin. These are similar to adhering junctions in epithelia but have a different name in muscle tissue.
- Desmosomes connect to intermediate filaments and provide mechanical support to the entire tissue
- Gap junctions allow the spread of current (ions) between adjacent cells and allow cardiac muscle to contract in a coordinated fashion

This image shows two cardiomyocytes by electron microscopy at two different magnifications. In the main image note sarcomeres and numerous mitochondria. Note the wavy dark line that connects the two cells. This is the intercalated disc. The inset shows the intercalated disc at higher magnification. Note that the intercalated disc runs perpendicular to the cells for most of the length of the intercellular junction but some point it turns and runs parallel. The parallel region contains gap junctions that allow current in the form of ions to pass between the two cells. By placing the gap junctions in the region of the intercalated disc that is oriented parallel to the cells, it reduces the tensile force of the gap junctions that increases every time the cells contract. Also, note how the sarcomere ends at the intercalated disc. The actin filaments of the sarcomere attach to the cadherins that link the adjacent cells.

Smooth Muscle
Smooth muscle surrounds most of the internal organs inner bodies and most blood vessels and veins. It provides tone and shape but can also generate slow and powerful contractions to change the size and shape of an organ. Smooth muscle is composed of numerous spindled shaped cells.

To get a better sense of how smooth muscle cells control the shape of an organ, one can look at blood vessels and bronchioles. Here, the smooth muscle cells are arranged circumferentially around the vessels and bronchioles. Contraction of the cells, decreases the diameter of the lumen of the vessel to restrict the volume of blood that can flow through vessel. The cardiovascular system uses smooth muscle to control the distribution of blood to different capillary beds.

Smooth muscle fibers are elongated spindle-shaped cells with a single nucleus. The nucleus is located centrally and the sarcoplasm is filled with myosin and actin filaments. Importantly, these filaments are not arranged into sarcomeres as they are in skeletal and cardiac muscle cells.

Smooth Muscle Orientations
The arrangement of smooth muscle differs from organ to organ. Usually, groups of smooth muscle cells will be oriented in one direction to provide contractile force in that direction. This image is a cross section of the ileum. The smooth muscle in the intestine is arranged into two layers. In the layer at the top of the image (layer 1), the smooth muscle cells are arranged to contract in the direction indicated. Note the nuclei in these cells are flat and elongated. The layer at the bottom of the image (layer 2) contains smooth muscle cells that are oriented to contract into and out of the plane of the screen. Note that the nuclei of these cells are round.

Smooth Muscle Cell Electron Micrograph
Myosin and actin filaments are scattered throughout the cytoplasm of smooth muscle cells. Actin filaments are attached to dense bodies on the cell membrane and within the cytoplasm. Dense bodies serve a similar function as Z-discs in skeletal and cardiac muscle cells. Activation of the myosin filaments pulls the dense bodies closer together causing the cell to shrink.

The electron micrograph shows an adhering junction between two adjacent smooth muscle cells. Similar to adhering junctions in other cells, cadherins mediate the interactions between cells and are linked to actin filaments within cells.

Smooth Muscle Innervation
Smooth muscle cells can be innervated in different ways. In multiunit smooth muscle, cells are individually innervated. Neurotransmitter from axons binds receptors on smooth muscle cells to initiate a signaling pathway that increases cytosolic calcium and muscle contraction. The signaling pathway does not appreciably change membrane potential. In unitary smooth muscle, one smooth muscle cell in a group is innervated and neurotransmitter initiates an action potential in that cell which propagates to neighboring cells through gap junctions.

For current to pass between smooth muscle cells, the cells are linked via gap junctions to allow ions to diffuse from one cell to another.

Similar to skeletal and cardiac muscle, increases in cytosolic calcium trigger contraction in smooth muscle, but the mechanism by which calcium stimulates contraction differs in smooth muscle cells. Instead of shifting tropomyosin on actin filaments to expose myosin-binding sites, calcium leads to the activation of myosin through an enzymatic pathway. When cytosolic calcium levels are low, muscle myosin is in an inactive state. A rise in cytosolic calcium bind to a protein called calmodulin. Calmodulin then activates an enzyme called myosin-light chain kinase or MLCK. MLCK phosphorylates light chains in myosin that activate the motor domain and lead to contraction.

Coupling cytosolic calcium to myosin activation via an enzymatic pathway has some important physiological consequences. First, smooth muscle cells take longer to initiate contraction and contractile force increases more slowly over time compared to skeletal and cardiac muscle. Second, smooth muscle cells can maintain contraction long after the stimulus is removed. This is due in part to the length of time it takes to inactive smooth muscle myosin. Inactivation of smooth muscle myosin requires the dephosphorylation myosin light chain, which is an enzymatic process and takes much longer than knocking myosin off of actin as is done in skeletal and cardiac muscle. A third factor allows smooth muscle to maintain contractile force is the slow rate at which muscle myosin to releases from actin filaments. Once bound to an actin filament, smooth muscle myosin remains attached even when cytosolic calcium concentration falls. This biochemical property of smooth muscle myosin allows it to maintain tension without consuming a lot of ATP.

Nervous System
Introduction
Our discussion of the nervous system will focus on the structure and function of the peripheral nerves.
Spinal Cord
Although the spinal cord is part of the central nervous system, many neurons with axons in peripheral nerves have their cell bodies in the spinal cord. In addition, peripheral nerves send projections into the spinal cord. Therefore, an understanding of the architecture of the spinal cord is important for understanding peripheral nerves.
Many important features of the spinal cord are visible in this cross section. The white matter is composed of nerve fibers carrying information to and from the brain and makes up the outer regions of the cord. The nerve fibers contain mostly myelinated axons which accounts for the light appearance of the region. The grey matter, which is located in the center of the cord, contains neuronal cell bodies and the axons and dendrites that emanate from those cell bodies. The presence of the cell bodies lends this region a darker color. The grey matter is divided into the dorsal (posterior) horn and ventral (anterior) horn. The ventral horn contains the cell bodies of motor neurons whereas the dorsal horn contains cell bodies of neurons that receive inputs from sensory neurons.
Attached to the spinal cord are two prominent structures: the dorsal root and ventral root. The dorsal root contains the cell bodies of sensory neurons. The ventral root contains the axons of motor neurons whose cell bodies reside in the ventral horn.

Attached to the spinal cord are two prominent structures: the dorsal root and ventral root. The ventral root contains the axons of motor neurons whose cell bodies reside in the ventral horn matter.
Dorsal Root
The dorsal root contains the cell bodies of sensory neurons that bring information from the periphery to the spinal cord. These neurons are pseudounipolar and contain an axon-like process that bifurcates with one branch extending toward the periphery and the other branch heading toward the grey matter of the spinal cord. Fibers heading toward the periphery leave the ganglion through the spinal nerve, where they run together with motor fibers. The dorsal root ganglion also contains satellite cells, which provide structural and metabolic support to the sensory neurons.

Motor Neurons
Motor neurons innervate one or many muscle fibers to control muscle contraction. Motor neurons are typically multipolar with an axon that terminates in a neuromuscular junction on the surface of skeletal muscle fibers. The dendrites of motor neurons branch and receive inputs from neurons in the spinal cord.
The motor neuron in the ventral horn is easily identifiable by its large size, polygonal shape and extension from the cell body. Compare the size of the nucleolus in the motor neuron with the nuclei in the surrounding support cells.

Another useful stain for neurons is Nissl which labels rough endoplasmic reticulum in neurons. The dark blue structures are referred to as Nissl bodies but are the equivalent of the rough endoplasmic reticulum. Note that the rough endoplasmic reticulum is confined to the cell bodies and dendrites and does not extend into the axons, which makes Nissl stain effective for differentiating dendrites and axons.

Peripheral Nerve Bundle
Peripheral nerves contain axons from several neurons and contain three layers of connective tissue. Connective tissue contains extracellular matrix and specific cells. Each axon in a peripheral nerve is associated with a Schwann cell that may or may not wrap the axon in myelin. The Schwann cells and surrounding extracellular matrix make up the endoneurium. Several axons are bundled into a structure called a fascicle. Each fascicle is wrapped by another layer of connective tissue called perineurium. Finally, the entire nerve is wrapped in a layer of connective tissue called the epineurium. Note that blood vessels are found in the perineurium but not the endoneurium.

Peripheral Myelinated Nerve Bundle
This image shows the three connective tissue layers of peripheral nerve that contains myelinated axons. Note that each axon is surrounded by a white space which is where the myelin sheath resides. Because myelin is mostly lipid, it stains poorly in histological samples. A thin layer of pink-staining connective tissue, endometrium, is visible around the myelin. The perineurium is a thicker layer of connective tissue that encases several axons. Finally, epineurium is the thickest layer of connective tissue and envelopes the entire nerve.

Myelinated Axon - Electron Micrograph
This electron micrograph shows a cross section of a myelinated axon in the peripheral nervous system. A Schwann cell surrounds the axon and wraps it in a sheath of myelin. Collagen fibers of the endoneurium surround the axon and Schwann cell.

Peripheral Unmyelinated Nerve Bundle
Nerves that carry unmyelinated axons contain the same three layers of connective tissue that are found in nerves with myelinated axons. The nuclei of Schwann cells are visible but the axons are difficult to see because they are not set off against the light staining of the myelin sheath. The perineurium and epineurium are clearly visible.

Unmyelinated Axons EM
Schwann cells still envelope unmyelinated axons but do not wrap them in myelin sheaths. This electron micrograph shows a Schwann cell associated with several small axons but without myelinating the axons. In unmyelinated nerves, Schwann cells can associate with several axons; whereas in myelinated nerves, Schwann cells associate with only one axon. Again, note the presence of collagen in the endoneurium.

Peripheral Nerve - Longitudinal Section
Viewed longitudinally, peripheral nerves appear to have a wave-like form. This arrangement lends peripheral nerves elasticity and extra length to be stretched when a limb is extended. The elongated nuclei within the nerve belong to the Schwann cells.

Peripheral Ganglion
Some organs contain collections of nerve cell bodies organized into ganglia. For example, the small intestine contains numerous ganglia located in its smooth muscle layers. The dendrites of the neurons in the peripheral ganglia receive impulses from nerves extending from the spinal cord, and the axons of the neurons synapse with cells in the organ to regulate their activity. Besides the cell bodies of neurons, peripheral ganglia also contain satellite or support cells.
