Structure and Function of Connective Tissue and Bone
Connective Tissue
Connective tissue comprises a collection of protein fibers and large sugars and several specialized cells. The fibers and sugars give connective tissue its mechanical properties while the cells provide specific functions.
Connective tissue can serve different functions. It provides mechanical support by resisting tension and compression. It organizes cells into tissues by binding to surface receptors on cells and regulating their growth and morphology. It provides metabolic support in the form of growth factors, hormones, and high energy lipids. It can also contain a variety of cells that generate immune responses to foreign objects. These functions tend to be exclusive so that connective tissue that is mechanically robust offers less metabolic and immune support. In contrast, connective tissue that provides metabolic and immune support tends to be weaker.

The mechanical strength of connective tissue varies widely, from the stiffness and hardness of bone to the squishiness of many organs. In between are types of connective tissue with different mechanical properties. Tendons resist tension and do not stretch making them ideal for linking muscle to bone. Cartilage resists compression. Large blood vessels can withstand stretch and recoil in response to changes in blood pressure. All of these mechanical properties are mediated by the components of connective tissue.

Several important proteins determine the mechanical properties of connective tissue and were described in the lecture on extracellular matrix. In general, these molecules either resist tensile and stretching forces or compressing forces. Collagen is the main component that resist tension. Elastic fibers also resist tension but behaves similar to rubber in that it can be stretched and will recoil after the force is removed. On the other side are glycosaminoglycans that resist compressive forces. Glycosaminoglycans are long sugar polymers that occupy large volumes within connective tissue.

Classification of Connective Tissue
Connective tissues are often divided into three classes based on the density and arrangements of collagen fibers. Dense regular connective tissue contains a lot of collagen and few cells. Also, the collagen fibers are arranged in parallel arrays along the lines of tension. This provides maximal resistance to external forces in one direction.
Dense irregular connective tissue also has a lot of collagen and few cells, but the collagen fibers that are less organized and oriented in multiple directions. This type of connective tissues allows the tissue to resist tension in multiple directions. Skin has dense irregular connective tissue.
Loose connective tissue has few collagen fibers and contains more proteoglycans, cells and vasculature. Loose connective tissue is common in organs that are not subject to mechanical stresses, such as the small intestine shown below. Loose connective tissue provides the cells in the organ with metabolic and immune support. Loose connective tissue is often referred to as ground substance in histological samples and appears as white space because the proteins and glycosaminoglycans stain poorly by H&E.

Several types of connective tissue don’t fit neatly into the above classification and are described separately.
Reticular Fibers
Reticular fibers are composed of type III collagen. Unlike the thick and coarse collagenous fibers, reticular fibers form a thin reticular network. Such networks are widespread among different organs and form supporting frameworks in the liver and lymphoid organs. Reticular fibers organize the cells in these organs and provide some mechanical support.

Adipose tissue
Adipose tissue is composed of adipocytes or white fat cells which are specialized for the storage of triglyceride. Individual adipocytes can be found in loose connective tissue but when aggregated into large groups, they form adipose tissue. Adipose cells store lipids and triglycerides in lipid droplets that grow in number and size that they push the rest of the cytoplasm toward the outside of the cell. Adipocytes can grow up to 100 µm. Adipose tissues also contains numerous capillaries.

Brown Fat Cells
Brown fat cells are highly specialized for temperature regulation. These cells are abundant in newborns and hibernating mammals, but are rare in adults. They have numerous, smaller lipid droplets and a large number of mitochondria, whose cytochromes impart the brown color of the tissue. The electron transport chain of these mitochondria is disrupted by an uncoupling protein, which causes the dissipation of the mitochondrial hydrogen ion gradient without ATP production. This generates heat.

Cells of Connective Tissue
Although the connective tissue has a lower density of cells than the other tissues you will study this year, the cells of these tissues are extremely important.
Fibroblasts
Fibroblasts are by far the most common native cell type of connective tissue. The fibroblast synthesizes the collagen, elastic fibers and proteoglycans of the extracellular matrix. These cells make a large amount of protein that they secrete to build the connective tissue layer. Some fibroblasts have a contractile function; these are called myofibroblasts. This electron micrograph shows several fibroblasts. Note the large nuclei, with heterochromatin and euchromatin, as well as the abundant rough endoplasmic reticulum. Fibroblasts can be seen by light microscopy in type of loose connective tissue called areolar tissue.

Mast Cells
Mast cells are granulated cells typically found in connective tissue. These cells mediate immune responses to foreign particles. In particular, they release large amounts of histamine and enzymes in response to antigen recognition. This degranulation process is protective when foreign organisms invade the body, but is also the cause of many allergic reactions. Mast cells can be identified in connective tissue by their numerous cytoplasmic granules.

Macrophage
Macrophages are phagocytic cells that are capable of engulfing foreign antigens and remnants of dead cells. Macrophages descend from monocytes and are found in most organs where they have different names depending on the organ. This image shows a macrophage with an irregularly shaped nucleus. The cytoplasm of the macrophage contains phagosomes and residual bodies, which are lysosomes with undigested material. On the right is a macrophage in the airway in the lung. These macrophages are called dust cells.

Cartilage
Cartilage is a specialized form of connective tissue produced by differentiated fibroblast-like cells called chondrocytes. It is characterized by a prominent extracellular matrix consisting of various proportions of connective tissue fibers embedded in a gel-like matrix rich in glycoproteins and hyaluronan. Chondrocytes produce all of the structural components of cartilage, including collagen, proteoglycans and glycosaminoglycans. Note the basophilia of the cytoplasm and the presence of lipid droplets.

Three kinds of cartilage are classified according to the abundance of certain fibers and the characteristics of their matrix.
Hyaline Cartilage
Hyaline cartilage is the most common type of cartilage and has a matrix composed of type II collagen and chondromucoprotein, a copolymer of chondroitin sulfates A and C (a disaccharide) with protein. Its high concentration of negatively-charged sulfate groups makes it appear intensely basophilic under H&E, and it often has a glassy appearance. Note the numerous chondrocytes in this image, surrounded by the cartilage they have produced. These cells have relatively small nuclei and often demonstrate lipid droplets in their cytoplasm. The spindle-shaped cells in the perichondrium can differentiate into chondroblasts that will eventually develop into chondrocytes.

Fibrocartilage
Fibrocartilage is distinguished by its high content and orderly arrangement of type I collagen fibers. It is typically located in regions where tendons attach to bones, the intervertebral discs, and the pubic symphysis. Numerous chondrocytes are spaced between the fibers. Note that the chondrocytes are surrounded by a matrix which helps differentiate fibrocartilage from dense connective tissue.
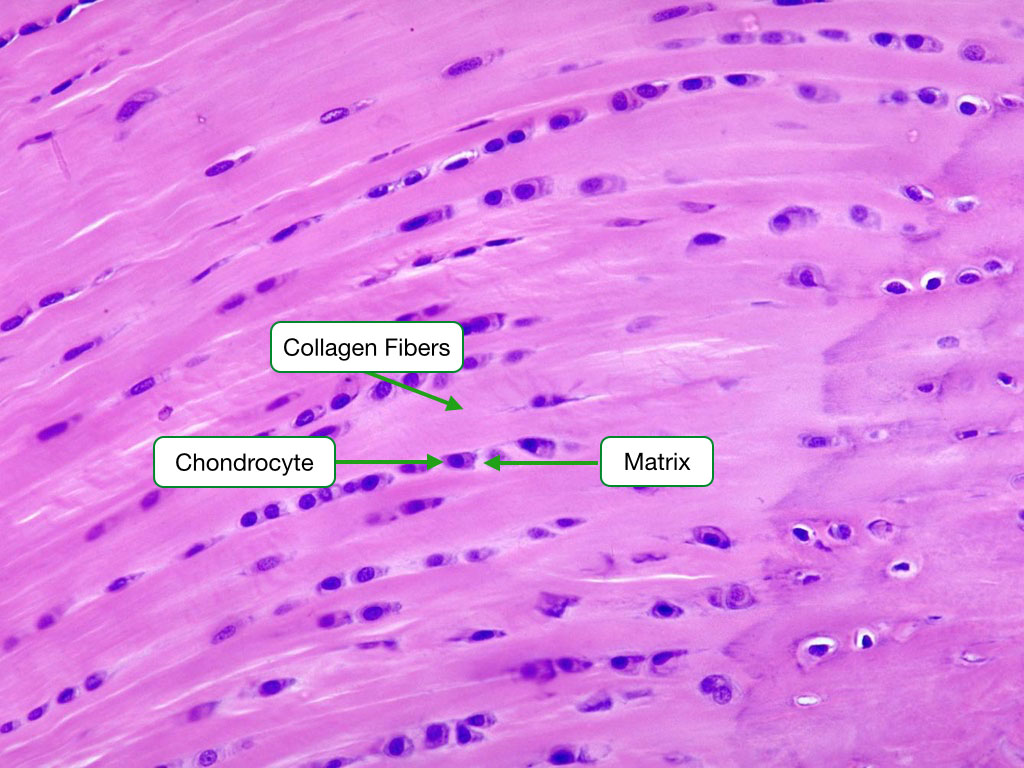
Elastic Cartilage
Elastic cartilage is characterized by the presence of abundant elastic fibers and is quite cellular. It is made up of type II collagen and is located in the auricle of the ear and the epiglottis. Verhoeff’s stain was used below to label elastic fibers.

Bone
Introduction
Bone is a form of connective tissues with special properties and serves several functions:
- Mechanical support for sites of muscle attachment
- Protection of vital organs
- Reservoir for calcium and phosphate
- Site for development of blood cells
Bone Composition
The composition of bone is quite simple as it is made of two major components that interact to form a composite material: type I collagen fibrils and crystal of calcium-phosphate. Calcium-phosphate contains a mixture of calcium and phosphate and are often called hydroxyapatite. During bone formation hydroxyapatite crystallizes along the length of a collagen fibril to form a mineralized fibril that is the main structural component of bone. Bone also contains several minor protein components many of which facilitate the formation of calcium-phosphate crystals on collagen fibrils.

The mineralized collagen fibrils can be arranged in several different patterns but the two that are most prominent are lamellar and woven. In lamellar bone the fibrils are arranged in parallel arrays. This arrangement gives bone maximal mechanical strength and is the type you will see in most fully developed bone. In woven bone the fibrils are arranged in random orientations with little organization giving the macro structure of woven bone a much more disorganized appearance than lamellar bone. Woven bone is the immature form of bone and is seen in fetal development. It is also found at sites of fractures where the bone cells rapidly synthesize woven bone as a temporary stabilizing and replacement for lamellar bone. Eventually, woven bone is replaced by lamellar bone to provide better structural support.

Architecture of Bone
Most bone comes in two architectures, both of which are seen in this section of long bone. On the outer surface is compact bone that appears as a dense wall of bone, but as we’ll see, compact contains blood vessels and nerves. In the interior is trabecular bone that consists of network of bone struts. Trabecular bone reduces the weight of bone while still providing robust mechanical support. The network of trabeculae absorb compressive force by bending. It also allows space for bone marrow and differentiation of blood cells. Because we are focusing on the mechanical functions of bone, it’s important to appreciate the structure and sections of long bone that are the primary load-bearing bones in the body.

Long bones, as the name implies, are longer in one direction and consist of a shaft with two knob-like structures at either end. The shaft is called the diaphysis and the ends epiphysis. The outer surface of the bone is called the periosteum and along the diaphysis is covered in a fibrous material. The epiphysis is covered with cartilage that cushions interactions with adjoining bones. The inner surface of compact bone is called endosteum or endocortical surface. The medullary cavity runs the length on the diaphysis and is the bone marrow is located.
Compact Bone
Compact bone consists of two arrangements of lamellar bone. The outer and inner surface are lamellae arranged circumferentially around the bone. These lamella circumnavigate the entire outer and inner surface of the bone. The second arrangement is called Haverisan systems or osteons. These are lamellae organized in a ring structure. In the center of the ring run blood vessels and nerves in Haversian canal. The canals run parallel to the long axis of bone. A second canal system, Volkmann’s canals carries blood vessels into and out of compact bone.

This image shows Haverisan systems in cross section. Note that lamellar bone is arranged around a central canal that contains blood vessels and nerves. Also, visible is a Volkmann’s canal that brings blood vessels and nerves from outside the bone.

Trabecular Bone
This image illustrates trabecular bone. Note the thin, branched spicules of bone. Within the bone matrix are osteocytes. Around the trbeculae is bone marrow.

Bone Cells
Osteoblasts
Osteoblasts are the cells that synthesize bone. Similar to fibroblasts they synthesize and secrete type I collagen that self-assembles into fibrils in outside the cell. Osteoblasts also secrete proteins that bind calcium and increase the amount of free phosphate, providing the raw materials for the formation of calcium-phosphate crystals. Over time the collagen fibrils will become coated with calcium-phosphate crystals to form bone.

Osteoblasts that are actively synthesizing new bone are easy to identify in histological sections because of the presence of osteoid. Osteoid is unmineralized collagen fibrils which stains lighter than bone. Over time the collagen fibrils calcify to become bone which stains darker.

Osteocytes
During the synthesis of bone, some osteoblasts will become trapped within the bone as it calcifies. These former osteoblasts are now called osteocytes. Osteocytes remain alive in the bone and perform many important functions. They are capable of resorbing and synthesizing bone and respond to signals to release calcium into the blood or store excess calcium. In addition, osteocytes appear to function as mechano-sensing cells. They detect and respond to mechanical stress by initiating changes in gene expression. This may lead to changes in the structure of bone that better resists the mechanical stress.

This image shows osteocytes (stained dark) in a Haversian system. Note the concentric layers of lamellar bone and osteocytes around the Haversian canal. Within the lameallar bone osteocytes maintain connections with each other through channels in bone called canaliculli.

Osteocytes extend processes called filopodia through the canaliculli. Filopodia from adjacent cells contact each other and communicate via gap junctions.

Osteoclasts
The third cell type is an osteoclast. Osteoclasts digest and resorb bone. They accomplish this by secreting hydrogen ions to lower the pH on the surface of bone which dissolves the calcium-phosphate crystals. Osteoclasts express on their cell surface the hydrogen ion pump that hydrolyzes ATP and uses the energy to move hydrogen ions from the cytosol to the surface of bone which is against a concentration gradient. The osteoclasts also express a chloride channel that allows cytosolic chloride to diffuse out of the cell. The net effect of the pump and channel is the secretion of hydrogen chloride onto the surface of bone.
Osteoclasts generate hydrogen ion by converting carbon dioxide and water to hydrogen ion and bicarbonate. Carbonic anhydrase catalyzes the reaction. The hydrogen ion is pumped onto the surface of bone and bicarbonate is exchanged for chloride.

Osteoclasts also secrete proteases that digest collagen fibrils. Note the ruffled border of the cell membrane increases the surface area for secretion. One important feature of of osteoclasts is that they form a sealing border around the bone to prevent the loss of hydrogen ions and proteases to the surrounding environment. This increases the efficiency on bone resorption and prevents damage to surrounding material. The sealing border is generated by interactions between integrins in the cell membrane of osteoclasts and fibers in bone.

After the bone is broken down by low pH and digestive enzymes, the digested material (e.g. amino acids, peptides) is endocytosed by osteoclasts. The endosomes are transported to the opposite of the osteoclast where they fuse with the plasma membrane to release their cargo. This process allows the components that make up bone to be recycled.

Osteoclasts are easy to identify in histological samples because they are multinucleated and are found in regions where bone appears to be carved out.

Dynamics of Bone
One of the misconceptions about bone is that it is a static material. Bone is highly dynamic and is constantly being made and resorbed. Synthesis can be due to the need to store excess calcium or increase the length of bone during development. Resorption can be triggered when blood calcium levels fall and calcium needs to be released from bone. The release and storing of calcium make minor changes in bone.

Bone is constantly being resorbed and replaced by new bone. This is most apparent in load-bearing bones where damage to the integrity of bone can accumulate because of daily stress. This old, damaged bone is resorbed and replaced with new, undamaged bone. It’s similar to the streets that accumulate cracks and damage and have to be dug up and resurfaced on a continuous basis. What’s critical to the mechanical integrity of bone is to maintain a proper balance between resorption and synthesis. If the balance swings too far to one side, bone loses its mechanical integrity. For example, osteoporosis is thought to be a loss of balance in which resorption outpaces synthesis.
Osteoblasts and osteoclasts are involved in two main processes within bone: bone modeling and bone remodeling. Bone modeling reshapes bone to increase the mechanical strength of bone and to react to changes in the amount or direction of mechanical stress. Bone remodeling replaces old, damaged bone with new bone to maintain the mechanical integrity of bone. Osteoblasts and osteoclasts are involved in both processes and the primary difference is that in bone modeling osteoblasts and osteoclasts work on opposite surfaces of bone, whereas in bone remodeling they work on a common surface.

Bone Modeling
This image illustrated the process of bone modeling on trabecular bone. Note that the osteoclasts (OC) and osteoblasts (OB) are working on opposite sides of the trabeculae. The net effect will be to move the trabeculae to the right and reorient its axis. This may occur in response to changes in the direction of mechanical stress.

Note that the trabeculae align along a couple of axes. The alignment of trabeculae corresponds to the direction of compressive forces. The ability of bone to align and position trabeculae is due to bone modeling.

Bone modeling also acts on compact bone to increase the stiffness of bone. As we grow, our long bones increase in diameter to provide more stiffness to withstand the larger stresses we generate. Although our bones need to increase in diameter, they also need to limit their weight to allow us to move more easily and with less energy. Bone modeling accomplishes this by growing the diameter of bone but limiting the increase in mass. Osteoblast on the periosteal surface lay down new bone to increase the diameter. Osteoclasts on the endosteal surface absorb bone to limit the mass. The result is bone with larger diameter but where the thickness of compact bone increases slightly or remains constant. The result is bone that is stiffer because of the larger diameter of the bone but with minimal gain in weight.

Mechanical stress on bone can also initiate bone modeling. Osteocytes appear to be the bone cells that senses and responds to mechanical stress. Compressive forces cause fluid to ebb and flow through the canaliculli. Osteocytes detect that flow of fluid through a structure called a primary cilium that extends from the cell membrane of the osteocyte. Movement of the cilium triggers signaling pathways in the osteocyte that increases cytosolic calcium and triggers changes in gene expression. The products of these genes can stimulate osteoclasts and osteoblasts to initiate bone modeling. Recall that osteocytes are linked via gap junctions so a rise in cytosolic calcium in one osteocyte can propagate to neighboring osteocytes.

Bone remodeling
The second process involving osteoblasts and osteoclasts is bone remodeling where old bone is replaced with new bone. Over time the mechanical stress imparted on bone generates small fractures or microcracks, revealed by the red dye. On their own these fractures are not sufficient to affect the mechanical integrity of the bone, but if allowed to accumulate, they could reduce the integrity to the point that makes the bone susceptible to larger fractures. Bone remodeling eliminates these small fractures by resorbing old bone and replacing it with new bone.

There are three phases to bone remodeling. The phases are illustrated on the surface of trabecular bone. First, is activation and resorption where osteoclasts become activated (more about this later) and begin to resorb old bone. Note the multinucleated cell is an osteoclast. The second phase is reversal in which the osteoclasts have finished cutting away the old bone and undergo apoptosis. Finally, during formation, osteoblasts synthesize new bone to replace the old bone removed by the osteoclasts. Note the lighter stained osteoid that will eventually calcify to form bone. Also, note that osteoclasts and osteoblasts are working on the same surface.

Bone remodeling also occurs in compact bone and uses the same cells and phases as in trabecular bone. In compact bone, bone remodeling proceeds through tunnels in old bone. The remodeling is driven by what is termed a basic multicellular unit that consists of osteoclasts and osteoblasts. The osteoclasts are in front resorbing old bone and are easily identified because of they have several nuclei. The osteoblasts trail behind and synthesize new bone to replace the old bone. Also, note the presence of blood vessels in the tunnel. These vessels will remain in the tunnel and the tunnel will eventually develop into a Haversian canal surrounded by lamellar bone.

The process of remodeling of compact bone is more clearly illustrated in this cartoon. The longitudinal section reveals the resorption zone at the leading edge of the tunnel followed by a reversal zone where the osteoclasts are inactive. The formation zone consists of osteoblasts that synthesize new bone. The new bone is made in circular lamellae that will surround the blood vessel in the center of the tunnel. Eventually, the tunnel will be filled in with bone and become a new Haversian system. The images on the right illustrate how the Haversian systems evolve over time due to bone remodeling. Remodeling erodes away a portion of an old Haversian system and replaces it with a new one. The circumferential lamellar lines of the new Haversian system are all visible whereas the some of the lines of the old system have been removed.

Because bone is constantly undergoing resorption and synthesis, one risk is that if one process overtakes the other, there could be a loss of mechanical integrity. Although there are rare diseases where the rate of bone resorption falls below the rate of synthesis, the most common imbalance is where resorption outpaces synthesis. This leads to thinning of the bones and in the elderly osteoporosis.
Osteoclast Development
When thinking about the causes of osteoporosis and potential treatments, the focus has been on how osteoclasts are activated. Because osteoclasts initiate resorption, the thinking has been that over activation of osteoclasts leads to an imbalance between resorption and synthesis. Osteoclasts are derived from monocytes that circulate in the blood. Macrophage colony stimulating factor triggers differentiation into preosteoclasts. They are recruited into bone where they differentiate into osteoclasts via RANK-ligand. RANK-ligand is expressed on the plasma membrane of stromal cells and osteoblasts in bone. RANK-ligand binds the RANK receptor in the plasma membrane of osteoclast precursors. Activates gene expression that leads to differentiation into osteoclasts, including cell fusion to form multinucleated cells.

Osteoblasts also inhibit the differentiation of osteoclast precursors into osteoclasts by producing a soluble form of RANK receptor called osteoprotegerin or OPG. OPG resembles the RANK receptor but is a soluble protein secreted by osteoblasts. OPG binds to the RANKL on osteoblasts and prevents it from binding RANK receptor on osteoclasts. This prevents activation of RANK-receptor by RANK-ligand.

When blood calcium levels fall, cells of the parathyroid release a hormone called parathyroid hormone (PTH). PTH will trigger the resorption of bone to generate calcium. PTH increases the formation of active osteoclasts by increasing the expression of RANKL on osteoblasts. In addition, PTH also decreases the expression of OPG. The combination of higher RANKL and lower OPG allows for more efficient activation of osteoclasts by osteoblasts.

Decline in estrogen levels after menopause is associated with loss of bone density. There is evidence that estrogen both directly and indirectly affects the activity of osteoclasts. Estrogen appears to directly affect osteoclasts by inducing them to undergo apoptosis. In the absence of estrogen, osteoclasts will have a longer lifetime and resorb more bone, leading to a loss in bone mass. Estrogen also indirectly affects the activation of osteoclasts. First, estrogen appears to stimulate the production of OPG by osteoblasts. Recall that OPG reduces osteoclast activation, so lower estrogen results in lower OPG and more activated osteoclasts. Second, estrogen reduces expression of RANK-ligand on osteoblasts. Recall the RANK-ligand stimulates osteoclast development, so lower estrogen increase osteoclast activation.

Development of Bone
During development, bone can generally only form on a preexisting structures. The two most common structures are mesenchymal tissue and cartilage. Bone forming on mesenchyme is called intramembraneous ossification, whereas bone forming on cartilage is called endochondrial ossification. These are important not only during development, but the same mechanisms are used to repair bone when it fractures.
Intramembraneous Ossification
Intramembranous ossification is the direct conversion of embryological mesenchymal tissue to bone. The process begins when mesencyhmal cells differentiate into osteoblasts, which begin to synthesize osteoid that will eventually mineralize into bone.

Endochondrial Ossification
In endochondrial ossification, bone is synthesized over a cartilage template. This image shows a growing tibia. The purple growth plate is composed of cartilage synthesized by the embedded chondrocytes. Over time, the cartilage becomes calcified; the dark purple areas within the trabecuale are remnants of calcified cartilage. As the cartilage emerges from the growth plate, woven bone, which appears light blue in this slide, is laid down over the calcified cartilage. This preliminary bone will eventually be replaced through bone remodeling to produce more organized lamellar bone. Osteoid, appearing red, can be seen laid down over the primary trabeculae of woven bone with a cartilage core.

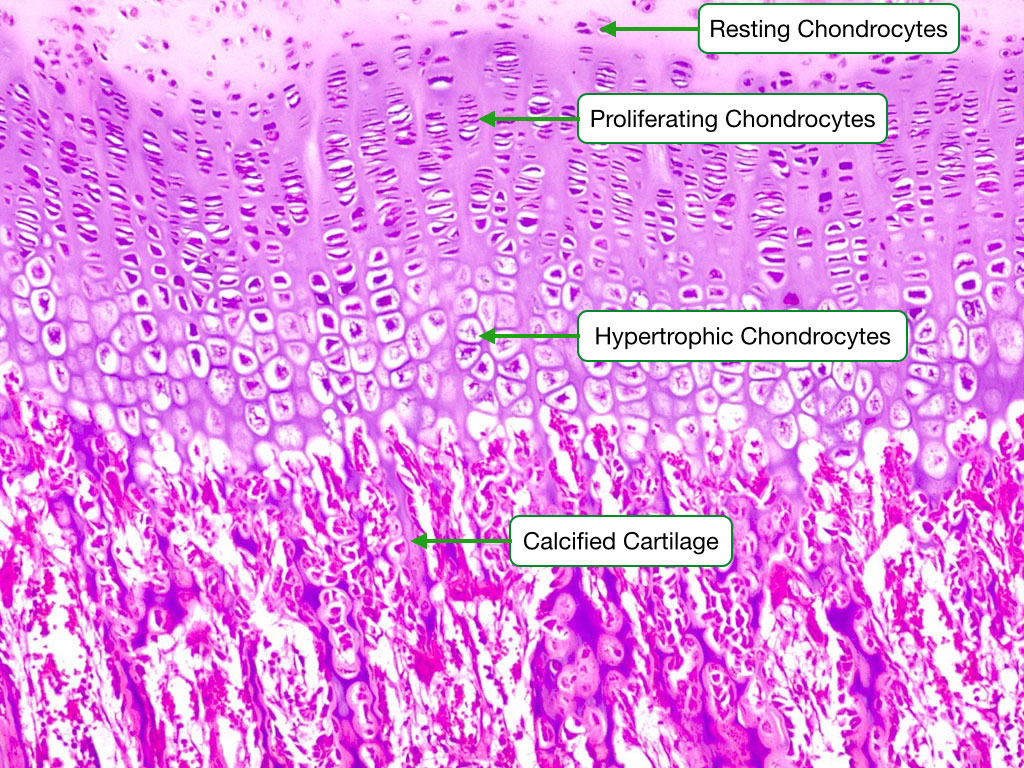
Chondrocyte Growth Sequence
This image shows the different stages of chondrocyte development during endochondrial ossification in a growth plate. At the top of the image are resting chondrocytes. Below them are chondrocytes in a stage of proliferation, during which the chondrocytes rapidly divide. This is followed by maturation/hypertrophy phases in which the chondrocytes increase in size and increase the rate of synthesis of cartilage. This cartilage is eventually calcified. Eventually, the calcified cartilage will be adsorbed by osteoclasts and replaced with bone.