Digestive Organs
Introduction
Although the gastrointestinal tract mechanically digests food through grinding and mixing, the cells of its epithelium provide limited enzymatic digestion beyond pepsin from the stomach and a few digestive enzymes on the surface of enterocytes in the small intestine. Instead, the gastrointestinal tract relies on different organs to secrete digestive enzymes and other molecules that breakdown and solubilize macromolecules in food. These organs include the salivary glands, pancreas and liver.
Exocrine Pancreas
The pancreas comprises two functional units. The largest is the exocrine portion that secretes digestive enzymes and bicarbonate which are delivered to the duodenum. The second is the endocrine pancreas that secretes hormones that regulate plasma glucose levels (e.g. insulin, glucagon, etc.) and will be described in the session on the Endocrine System.
The exocrine portion of the pancreas can be distinguished from the endocrine portion in H&E-stained samples The exocrine cells stain darker and are arranged in clusters called acini (described in detail below). The endocrine cells stain lighter and cluster in islands, called islets of Langerhans, admidst the exocrine cells.

Secretions from the exocrine pancreas are delivered to the lumen of duodenum via a duct. The digestive enzymes breakdown macromolecules in chyme that comes from the stomach and the bicarbonate neutralizes the acid from the stomach. Enzymes from the pancreas are stored and secreted in two forms. Some enzymes are active upon release whereas others are inactive and are converted to active enzymes in the duodenum. The inactive enzymes are called zymogens.

The exocrine pancreas has two main cell types. Acinar cells synthesize and secrete digestive enzymes and duct cells that mainly secrete fluid rich in bicarbonate. The acinar cells form a small cluster called an acinus with their apical surfaces facing a central lumen. The lumen of the acinus leads into a duct defined by the duct cells. A group of acini form a lobule. Ducts from different acini in a lobule merge to and empty into interlobular ducts.

Pancreatic Acinar Cells
The acinar cells primarily secrete a set of digestive enzymes that will be delivered to the duodenum. The digestive enzymes fall into two classes: zymogens and active enzymes. Zymogens are enzymes that are synthesized in an inactive form and are converted to active form through proteolytic removal of a domain within the protein that keeps the enzyme inactive. Activation of zymogens occurs in the duodenum where an enzyme called enterokinase resides in the apical membrane of enterocytes in the epithelium. Enterokinase cleaves trypsinogen to form trypsin which is an active protease. Trypsin then removes the inhibitory domains from the other zymogens. The array of digestive enzymes breaks down macromolecules in chyme.
The zymogen or inactive form of the digestive enzymes is critical for protecting the pancreas. Premature activation of zymogens inside acinar cells or in the ducts digest cellular proteins leading to cellular damage and triggering inflammation.

Acinar cells store the digestive enzymes in cytosolic granules called zymogen granules and release digestive enzymes at a very slow rate when not eating. Three molecules increase the rate of release during eating: acetylcholine, gastrin and cholecystokinin (CCK). Neurons from the vagal nerve release acetylcholine during the cephalic phase when one smells or thinks about food. G-cells in the stomach release gastrin during the gastric phase when food enters the stomach. Last, enteroendocrine cells in the small intestine release CCK during the intestinal phase when food enters the duodenum.
All three molecules bind different G-protein coupled receptors. These receptors activate pathways that increase the activities of protein kinase A or protein kinase C and increase cytosolic calcium. The activated kinases and high cytosolic calcium levels trigger fusion of zymogen granules with the cell membrane to release the enzymes into the lumen space. The activate kinases also trigger synthesis of digestive enzymes to maintain a pool of digestive enzymes in zymogen granules.
In addition to releasing digestive enzymes, acinar cells also secrete a plasma-like fluid. Chloride channels in the apical membrane allow cytosolic chloride to flow into the lumen. The resulting lumen negative transepithelial voltage draws sodium into the lumen through the tight junctions. Water flows into the lumen through cellular junctions and aquaporin channels. To support chloride secretion, acinar cells contain NKCC channels in their basolateral membrane to increase cytosolic chloride concentrations. The sodium potassium pump and potassium channels maintain sodium and potassium gradients across the basolateral membrane.
Pancreatic Duct Cells
In addition to digestive enzymes, the pancreas also secretes bicarbonate which neutralizes acid in the duodenum. The cells that line the ducts secrete bicarbonate into the lumen through the chloride-bicarbonate exchanger. The CFTR channel in the apical membrane allows chloride to recycle back into the lumen. Duct cells generate bicarbonate through carbonic anhydrase converting water and CO2 into hydrogen ion and bicarbonate. The hydrogen ion is secreted across the basolateral membrane through the sodium-hydrogen ion exchanger and hydrogen ion pump. Duct cells can also take up bicarbonate across the basolateral surface through the sodium-bicarbonate co-transporter.

Two molecules increase the rate of bicarbonate secretion in duct cells: secretin and acetylcholine. Secretin is produced by enteroendocrine cells (S cells) in the duodenum in response to decreases in pH caused by fluid from the stomach. Secretin binds a G-protein coupled receptor that increases cytosolic cAMP and active protein kinase A resulting in increased rates of bicarbonate secretion. The parasympathetic nervous system releases acetylcholine which stimulates a pathway leading to active protein kinase C.
Histology of Acinar and Duct Cells
This H&E section of the exocrine pancreas shows several of its characteristic features. The acinar cells are arranged in clusters called acini and show a strongly basophilic cytoplasm that represents the area occupied by the rough endoplasmic reticulum. The apical side of the cells is filled with zymogen granules that contain the zymogens and active enzymes. Fusion of the granules with apical cell membrane releases zymogens and active enzymes into the lumen
From the lumen of acini, secreted proteins and fluid flow into intercalate ducts. A simple cuboidal epithelium lines the duct and secretes bicarbonate into the lumen of the duct through a chloride-bicarbonate exchanger in their apical cell membrane. The duct cells are cuboidal with place cytoplasm Also visible are centroacinar cells that form the terminal lining of the intercalated ducts.
A thin layer of connective tissue surrounds the acini and contains blood vessels and nerves.

Fluid from intercalated ducts flows into interlobular ducts and then finally into the pancreatic duct. Outside the pancreas, the pancreatic duct merges with the common bile duct that delivers bile from the liver and gall bladder. The common bile duct empties into the duodenum.
Pancreatic Acinar Cells EM
This electron micrograph shows in detail the acinar cells of the pancreas. These cells have basally located nuclei and numerous zymogen granules at their apical pole. They also have abundant endoplasmic reticulum in the basal portion of the cytoplasm.

Salivary Glands
Salivary glands secrete saliva, a lubricant composed of mucus, lysozyme, antibodies, inorganic ions, and amylase. Saliva is released in response to parasympathetic stimulation, and up to 1500 milliliters can be produced each day. The most important component of saliva for digestion is amylase, which hydrolyzes dietary carbohydrates into disaccharides.There are three major pairs of salivary glands: parotid, sublingual and submandibular.
All three salivary glands have a similar architecture to the exocrine pancreas with glands organized into tubulo-acinar structure. Recall, in a tubulo-acinar gland secretory cells are arranged in clusters called acini. The secretory cells in an acinus release enzymes and fluid into shared lumen that drains into an intercalated duct. Epithelial cells lining the duct can adjust the electrolyte composition and pH of the fluid. Intercalated ducts feed into interlobular ducts that lead to the main duct of the organ. Although the structure of the three salivary glands is similar, each secretes saliva of a slightly different composition based upon its primary cell types.
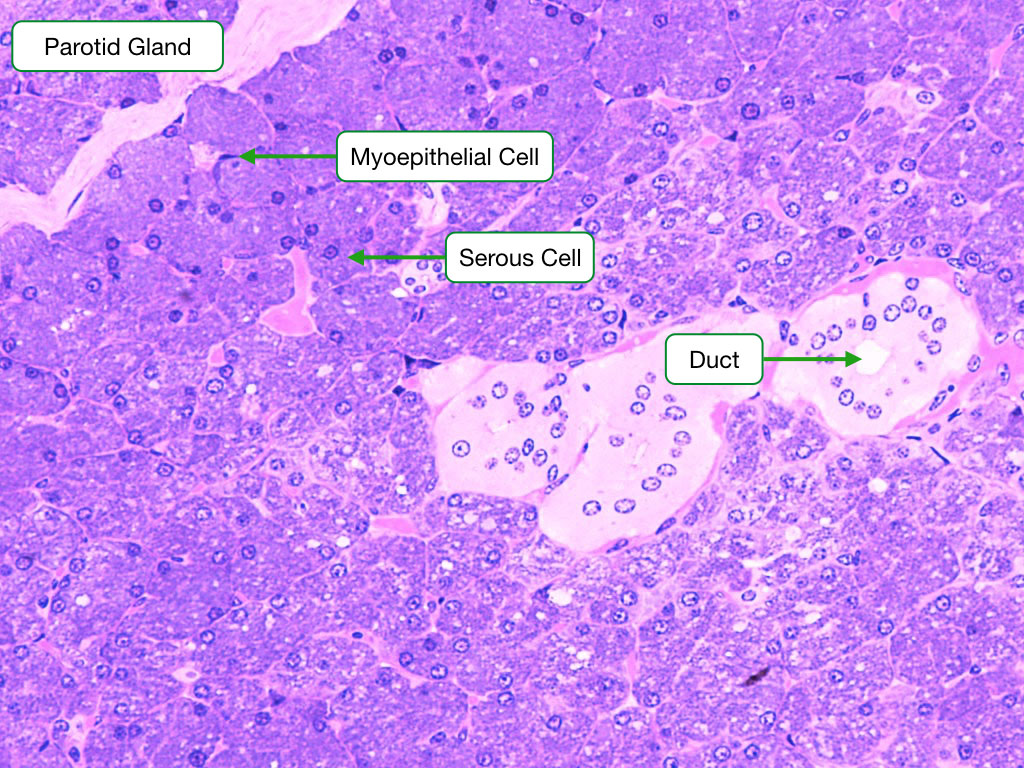
Parotid Gland
The parotid gland produces saliva that is watery and rich in enzymes (amylase and lysozyme) and antibodies. Two types of cells are visible in this section. The abundant serous exocrine cells make up the bulk of the gland and synthesize the enzymes. The cells are arranged into clusters called acini. The cells secrete protein at their apical surface into a central lumen within the acinus. The exocrine cells in the acini are surrounded on their basal side by contractile myoepithelial cells; when they contract, they squeeze the saliva out of the acinar lumen into the ducts that transport saliva out of the gland. The ducts in the parortid and other salivary glands are lined by a simple cuboidal epithelium. The cells in the epithelium actively absorb sodium to produce a hypotonic saliva.
Sublingual Gland
The sublingual gland is composed primarily of mucus cells. Similar to the parotid gland, the mucus cells are arranged into acini. Note the presence of a serous cell (serous demilune) that caps some of the acini. These cells produce lysozyme which digests the cell walls of bacteria. Although the serous demilune cells appear out of line with the mucus cells, this is an artifact of the fixation and preparation of the sample. In fact, the demilune cells are part of the same epithelium as the mucus cells.

Submandibular Gland
The submandibular gland is composed of both serous acini and mucus cells, and secretes saliva that contains more mucus than that of the parotid.

The ducts in all three glands are primarily responsible for adjusting the ionic composition of saliva. Sodium and chloride are absorbed to generate a hypotonic solution.
Liver
The liver is the largest organ of the body and performs several essential functions. Most of the functions are performed by a single cell type called hepatocytes. The liver secretes products that aid in the digestion of macromolecules and absorption of nutrients in the small intestine. Hepatocytes produce bile that is released into the duodenum via the common bile duct. Bile performs two major functions. First, it contains molecules that help solubilize, digest and absorb lipids. Second, bile contains many waste products, including bilirubin and excess cholesterol.
The liver also processes many of the nutrients that are absorbed by the small intestine. Amino acids and sugars that are absorbed by epithelial cells in the small intestine perfuse into fenestrated capillaries in the lamina propria. These capillaries coalesce into the portal vein that leads directly to the liver. Blood in the portal vein directly perfuses hepatocytes in the liver exposing them to the small molecules absorbed in the small intestine. The exceptions to this pathway are triglycerides, lipids and cholesterol which are packaged into chylomicrons by the enterocytes. Chylomicrons are too large to pass across fenestrated capillaries but instead enter the lymphatic system which bypasses the liver.
The liver plays a central role in homeostatic control of levels of carbohydrates, lipids and blood proteins in the body. Hepatocytes store large amounts of glycogen and lipid droplets that contain lipids, cholesterol and triglycerides. If plasma levels of glucose or lipid or cholesterol fall, hepatocytes release these components from their internal stores. Hepatocyes also synthesizes plasma proteins, such as albumin and coagulation factors.
The liver is also critical for metabolizing plasma proteins and detoxifying drugs that have been ingested through the GI tract. For example, old red blood cells are phagocytosed by liver macrophages which metabolize heme into bilirubin. Hepatocytes take up bilirubin, modify it and then excrete it as part of bile into the duodenum. Hepatocytes can biotransform many compounds including toxic drugs through a multistep process that involves either oxidation or reduction of the compound followed by attachment of molecule that increases the water solubility of the compound. Hepatocytes then excrete the metabolized compound into bile.
Structural Organization of the Liver
Hepatocytes are arranged in lobules which in cross section appear hexagonal. At the points of the lobule are a structure called the portal triad which contains the hepatic artery (freshly oxygenated blood), portal vein (blood from small intestine) and the bile duct. At the center of each lobule is the central vein. Hepatocytes reside between the portal triads and central vein.

Lobules define the histological arrangement of hepatocytes, but functionally, hepatocytes are separated into zones based on their location relative to portal triads. Hepatocytes in zone 1 are closest to portal triads and consequently receive blood with the highest concentrations of oxygen and nutrients. As the blood percolates into zones 2 and 3, the concentration of oxygen and nutrients decreases. Consequently, hepatocytes in zone 3 receive less oxygen and fewer nutrients than those in zone 1.

Blood and bile flow in opposite directions between portal triads and central vein. Blood (red arrows) moves from the hepatic artery and portal vein toward the central veins, where as bile (yellow arrows) flows from hepatocytes towards the portal triads.
The hepatocytes in the three zones have different metabolic activities and perform different functions. For example, hepatocytes in zone 1 are more prominently involved in gluconeogenesis and cholesterol synthesis, whereas hepatocytes in zone 3 are more active in glycogen synthesis. The cells in different zones also perform different steps in the biotransformation of drugs.
The image below is of a section of liver stained for glycogen. Note the cells closer to the central vein in zone 3 stain darker than those in zone 1 closer to the portal triad.

Portal Triads
Portal triads are composed of three major tubes. Branches of the hepatic artery carry oxygenated blood to the hepatocytes, while branches of the portal vein carry blood with nutrients from the small intestine. The structure of these blood vessels is similar to those in other organs, but note that the portal venule is much larger than the hepatic artery and consequently the hepatocytes receive more partially oxygenated blood than fully oxygenated blood. The bile duct carries bile products away from the hepatocytes, to the larger ducts and gall bladder. The bile duct is lined by a simple cuboidal epithelium.

Hepatocytes and Sinusoids
Blood from the hepatic artery and portal venule mixes and then flows toward the central vein through special vessels called sinusoids. Sinusoids are lined by a discontinuous endothelium. Interactions between hepatocytes creates a bile channel called a canaliculus through which bile flows in the opposite direction as blood toward the portal triad and into the bile duct. Stellate or Ito cells are found in the sinusoids with higher concentrations near the portal triads. Stellate cells have an important role in the liver’s response to damage. Under normal conditions, stellate cells are in a quiescent state. When the liver is injured, stellate cells are activated and begin to synthesize components of the extracellular matrix.

This H&E image shows the close proximity between the blood in the sinusoids and hepatocytes. It is crucial to understand the membrane topology of the hepatocyte: the apical surface of the hepatocyte is where bile secretion occurs and faces the lumen of the bile canaliculus; the basolateral surface of the hepatocyte faces the sinusoid and is where materials are absorbed from and secreted into the blood.

Also visible is a Kupffer cell. Kupffer cells are the resident macrophages of the liver and are typically found within the lumen of the sinusoids. Kupffer cells primarily phagocytose bacteria and old red blood cells. Heme from red blood cells is metabolized into iron and bilirubin.
Sinusoid EM
The sinusoids have a discontinuous endothelium with large gaps and no basement membrane. Consequently, sinusoids are permeable to most macromolecules including proteins and lipoproteins. There is a gap between the endothelium and the hepatocytes known as the space of Disse. The blood enters this space and percolates around the basolateral surface of hepatocytes. Hepatocytes contain numerous receptors and transporters that allow them to take up macromolecules, bile salts, nutrients and ions. Blood then flows from sinusoids into a central vein which eventually drains into the hepatic vein.

Hepatocyte EM
Hepatocytes secrete bile through transporters along their apical surface. The apical surfaces of adjacent hepatocytes form canaliculi that collects bile and allows it to flow toward the portal triad and into the bile duct. Tight junctions between adjacent hepatocytes keep the contents of bile in the canaliculi. From the bile duct, bile flows into the biliary tree which ends in the common hepatic duct. Outside the liver the common hepatic duct merges with the cystic duct coming from the gall bladder to form the common bile duct. Bile flowing from the liver can either be stored in the gall bladder or delivered to the duodenum through the common bile duct.

Gall Bladder
The gall bladder stores and concentrates bile from the liver. It has several important characteristic features that can be used to distinguish it from other organs in the GI system. These include irregularly shaped villi that are lined by abnormally tall columnar epithelial cells. The smooth muscle in the wall of the gall bladder contracts under the influence of the hormone cholecystokinin to expel the bile into the duodenum.
