Female Reproductive System
Introduction
The organs of the female reproductives system undergo structural changes in a cyclical pattern called the menstrual cycles. Four hormones are the primary drivers of changes during the menstrual cycle: follicle-stimulating hormone (FSH), leutenizing hormone (LH), estrogens and progesterone. The levels of these hormones fluctuate during the menstrual cycle to coordinate the structural and functional changes in the ovary and uterus. The hypothalamus and pituitary are the master controllers of hormone levels during the menstrual cycle. This reading will describe the histological changes in the ovary and uterus throughout the course of the menstrual cycle and the structure and function of the hypothalamus and pituitary that produce the hormones that drive these changes. The reading will also describe the tubes that connect the ovary and uterus and the uterus with the external environment.
Hypothalamus - Pituitary - Gonad Axis
Recall from the reading on the Endocrine System the central role the hypothalamus and anterior pituitary play in controlling the activities of other organs and the production of hormones in these organs. The hypothalamus and anterior pituitary play a similar role in controlling the timing of the menstrual cycle and the structural changes that occur.
The anterior pituitary contains cells that produce different types of hormones. Gonadotrophs are cells that synthesize and release two hormones, follicle-stimulating hormone (FSH) and leutenizing hormone (LH), that will directly regulate the production of estrogens and progesterone in the ovary. FSH and LH contain an alpha subunit and a beta subunit. The alpha subunit is the same in both FSH and LH, but the beta subunits differ.
Neurons in the hypothalamus stimulate the production of FSH and LH in gonadotrophs via the hormone gonadotropin-releasing hormone (GnRH). Hypothalamic neurons release GnRH in a pulsatile fashion (every 30 to 120 minutes) and the frequency of GnRH production can preferentially stimulate production of FSH or LH; higher frequency GnRH release favors LH production. The frequency of of GnRH release increases during the menstrual cycle which in part accounts for the changes in levels of LH and FSH over the course of the menstrual cycle. The hypothalamic neurons that produce GnRH are themselves regulated by other neurons to generate pulsatile releases of GnRH.
The structural relationship between the hypothalamus and pituitary are shown in the slide below. Neurons in the hypothalamus release GnRH which diffuses into a fenestrated capillaries. These capillaries coalesce into portal veins that travel to the anterior pituitary and then ramify into fenestrated capillaries to allow GnRH to (and other releasing hormones produced in the hypothalamus) diffuse out of the circulatory system and into the parenchyma of the anterior pituitary where gonadotrophs and other hormone-secreting cells reside.

Gonadotrophs in the anterior pituitary contain G-protein coupled receptors that bind GnRH. Recall that the cytosolic surface of G-protein coupled receptors interact with heterotrimeric GTP-binding proteins. Upon binding GnRH, the receptors catalyze the alpha-subunit to bind GTP which then triggers activation of several downstream pathways that increase cytosolic calcium and activate both protein kinase A and C. Ultimately, these pathways increase production of FSH and LH and secretion of both hormones.
Prolonged exposure (days to weeks) to high levels of GnRH leads to reduced production of FSH and LH. In most G-protein-coupled receptor signaling pathways, inhibition of the pathway is often generated by inactivating the receptor or reducing the concentration of the receptor through endocytosis. This form of inactivation can be generated in minutes.
The GnRH receptor lacks the cytosolic domain that allows cells to inactivate the receptor. Thus, over short time periods high levels of GnRH do not lead to a decrease in FSH and LH production. Instead, long-term exposure to GnRH eventually inactivate downstream components of the GnRH receptor pathway (e.g. IP3 receptor) which reduces production of FSH and LH. Because this mechanism of inactivate takes longer than inactivating the receptor, it takes several days of exposure to GnRH before FSH and LH levels fall.
Ovary
The human ovary consists of an inner medulla and outer cortex with indistinct boundaries. The medulla contains the blood vessels and nerves, while the cortex is occupied by developing follicles. An ovarian follicle progresses through several distinct phases before it releases its ovum, and a cross-section of an ovary will reveal follicles in various stages of development. The next sections will describe the histological features of each stage of follicular development and the major functional changes in the cells compose follicles.

Primordial Follicle
During the first five months of fetal development, a finite number of primordial follicles form in the fetal ovary. These follicles consist of oocytes surrounded by a single layer of squamous granulosa cells. These primordial follicles remain in the process of the first meiotic division.

Early Primary Follicle
At the start of each menstrual cycle a limited number of primordial follicles are triggered to develop. The first apparent histological difference between primordial and primary follicles is the granulosa cells that surround the oocyte change from squamous to cuboidal. Functionally, the cells express a receptor for FSH and are stimulated by FSH. Consequently, these cells are also called follicular cells.

Gap junctions connect the granulosa cells to each other and connect the granulosa cells with the oocyte. Gap junctions allow the diffusion of small molecules between granulosa cells and the oocyte and some of these small molecules keep the oocyte arrested in meiosis.
Late Primary Follicle
The late primary follicle stage is reached when the granulosa cells proliferate into a stratified epithelium known as the zona granulosa. In addition, a thin band of glycoproteins that separates the oocyte and follicular cells is seen. This band of glycoproteins is called the zona pellucida. The zone pellucida enlarges as the follicle develops and will accompany the oocyte if it is released from the ovary. Proteins on the surface of sperm will bind to specific glycoproteins in the zona pellucida to aid fertilization.

Just outside the granulosa cells is a layer of cells called theca cells. These cells express a receptor to LH which stimulates the cells to proliferate and synthesize androgens. A thin basement membrane separates the granulosa cells from the theca cells.
Secondary Follicle
The characteristic feature that distinguishes secondary from primary follicles is the appearance of a follicular antrum within the granulosa layer. The antrum contains fluid which is rich in hyaluronan and proteoglycans. Note the increase in cell layers of the zone granulosa, the thicker zone pellucida, and larger oocyte. At this stage, the theca cells have separated into two distinct functional types. Theca interna cells are closest to the granulosa cells and contribute to the production of estrogen. Cells in the theca externa more closely resemble smooth muscle cells.
The production of estrogen requires both the cells of the theca interna and granulosa cells. Estrogens, like all steroid hormones, are produced from cholesterol through a multi-step process that requires several different enzymes. Neither the cells of the theca interna nor the granulosa cells contain all of the enzymes necessary to convert cholesterol into estrogens. Theca cells contain enzymes that catalyze the initial conversion of cholesterol into androgens but lack aromatase that carries out the final steps of converting androgens into estrogens. Consequently, androgens produced by theca cells diffuse into the granulosa cells which contain aromatase but lack the enzymes for the initial steps in estrogen synthesis. The theca cells are in a better position to catalyze the initial steps in estrogen synthesis because they are closer to blood vessels and can take up LDL to obtain cholesterol.

Graafian Follicle
The Graafian follicle is the stage after the first meiotic division has completed but before ovulation. The oocyte is now a 2N haploid. The follicle is characterized by a large follicular antrum that makes up most of the follicle. The secondary oocyte, having undergone the first meiotic division, is located eccentrically. It is surrounded by the zona pellucida and a layer of several cells known as the corona radiata. When released from the Graafian follicle and into the oviduct, the ovum will consist of three structures: oocyte, zona pellucida and corona radiata.

Corpus Luteum
After release of the ovum, the remaining cells of the granulosa and theca interna form the corpus luteum. The center contains the remains of the blood clot that formed after ovulation. Surrounding the clot are glanulosa lutein cells and on the outside theca lutein cells. These cells produce progesterone and to a lesser extent estrogen.
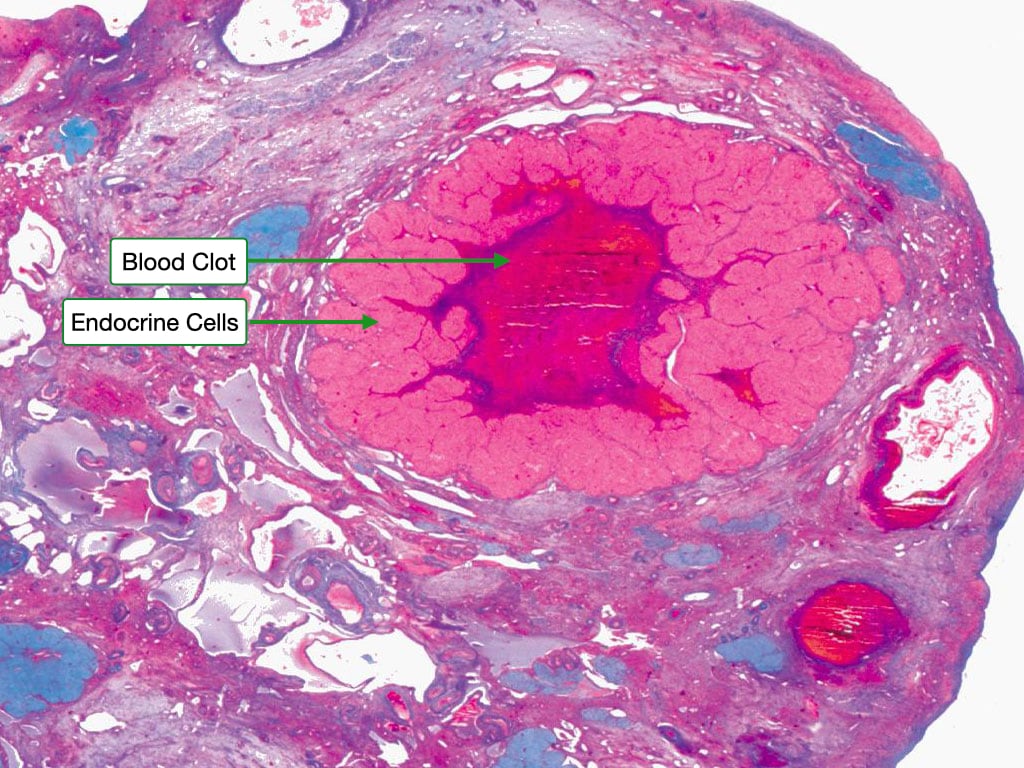
The granulosa lutein cells have an appearance characteristic of steroid-producing cells, with pale cytoplasm indicating the presence of lipid droplets. Theca lutein cells are smaller and more deeply stained. Blood vessels penetrate into region of the granulosa lutein cells allowing them to take up cholesterol to be used to synthesize progesterone.

The activity of the cells of the corpus luteum is sustained by leutenizing hormone. If the ovum is fertilized and implants in the uterine wall, human chorionic gonadotropin replaces leutenizing hormone to sustain the activity of the cells in the corpus luteum.
Corpus Albicans
If fertilization does not occur, the cells of the corpus luteum remain active for roughly 14 days until the levels of LH fall and the corpus luteum involutes to form the corpus albicans. The secretory cells of the corpus luteum degenerate, are phagocytosed by macrophages and replaced by fibrous material.

Atretic Follicle
Each menstrual cycle, several primordial follicles are stimulated to continue development but only one follicle completes development to release an ovum. The other follicles degenerate through a process called atresia which can occur at any stage of development. During atresia, granulosa cells undergo apoptosis and are replaced by fibrous material. The oocyte degenerates and the basement that separated the oocyte from granulosa cells thickens to become the glassy membrane. The decrease in FSH levels trigger atresia in all but the one dominant follicle.

Feedback to Control Hormone Levels
Positive and negative feedback play critical roles in regulating the concentrations of hormones during the menstrual cycle. An example of negative feedback is granulosa cells that are stimulated by FSH to secrete a set of hormones called inhibins. Inhibits act on gonadotrophs to reduce synthesis of beta-subunit of FSH.
Estradiol can generate negative and positive feedback. An example of negative feedback occurs in early stages of the menstrual cycle when estradiol reduced production of GnRH in the hypothalamus. Estradiol generates positive in the mid-cycle phase by increasing the expression of GnRH receptor in gonadotrophs. This makes the cells more sensitive to GnRH and leads in part to the surge of LH that triggers ovulation.
Oviduct
After release from the ovary, the ovum enters the oviduct. The oviduct consists of several segments: the infundibulum, which lies closest to the ovary, followed by the ampulla, the isthmus, and the pars interstitialis. The first two of these regions have a characteristic appearance that is dominated by an elaborate mucosa that is thrown into numerous branched folds, surrounded by a relatively thin layer of smooth muscle. As the tube moves away from the ovary and toward the uterus, these folds become smaller and the smooth muscle dominates.

Oviduct Epithelium
The oviduct epithelium consists of two distinct cell types. Ciliated cells dominate and serve to move the ovum away from the ovary and toward the uterus. Non-ciliated secretory cells, also known as peg cells, release a secretion that lubricates the tube and provides nourishment and protection to the traveling ovum.

Uterus
The uterus is divided into several layers that have distinct structural and functional characteristics. The simplest classification of these layers is their division into a mucosal layer, or endometrium, a muscularis layer, or myometrium, and a serosal layer, or perimetrium. The endometrium itself is divided into two layers, the stratum functionalis and stratum basalis. During the menstrual cycle, the stratum functionalis expands and vascularizes and is subsequently sloughed off during the process of menstruation, whereas the stratum basalis remains relatively constant. The myometrium allows for the expansion and contraction of the uterine cavity and is responsive to the hormone oxytocin.

Uterine Cycle
The endometrium undergoes dramatic structural and functional changes during the menstrual cycle. These changes are divided into two phases: proliferative and secretory.
Proliferative Phase
The proliferative phase is characterized by robust growth of the epithelial cells in the stratum functionalis and the formation of coiled and densely packed glands. This changes in this phase are driven by estrogen.


Secretory Phase
The secretory phase of the uterine cycle begins at ovulation. In this phase, the glands become even more complexly coiled and the endometrial lining reaches its maximal thickness, whereas the stratum basalis and myometrium remain relatively unchanged. Note the saw-toothed appearance of the glands. Secretions rich in glycogen and glycoprotein can be observed in the lumina of the glands. This phase is drive by progesterone.

Menstrual Phase
If fertilization does not occur, the placental tissue does not produce hCG and the corpus luteum in the ovary degenerates. Consequently, the levels of progesterone decrease, causing the spiral arteries in the endometrium to constrict and the tissue to become ischemic. This leads to cell death and the sloughing of the stratum functionalis.

Cervix
The cervix lies at the base of the uterus and serves to protect it from bacterial infiltration. It is the site of an important epithelial transition. The upper cervix (endocervix) is lined by a simple columnar epithelium that contains mucous-secreting cells. In contrast, the lower cervix (ectocervix) is lined by a stratified squamous epithelium. The transition point between these two epithelia is known as the external os. Note how the underlying layers of the cervix are composed primarily of collagenous and elastic connective tissue rather than smooth muscle fibers.

Vagina
The vagina is lined by a stratified squamous epithelium that features a small degree of keratinization. Below the epithelium is a thick layer of dense connective tissue, like that in the dermis of the skin. A layer of loose connective tissue containing many blood vessels and nerves follows this. The cells of the vaginal wall typically contain a relatively large amount of cytoplasm because they store and release glycogen. Glycogen is metabolized by commensal bacteria to lactic acid that prevents growth of pathogenic microorganisms.
