Female Reproductive System
Introduction
Roughly every four weeks, the female reproductive system releases one (usually) egg and builds an environment to accommodate an embryo should that egg be fertilized. To generate an egg and construct a potential home for an embryo, the ovaries and uterus of the female reproductives system undergo a cyclical pattern of structural changes called the menstrual cycle. Changes in the ovary are called the ovarian cycle and changes in the uterus are called the endometrial cycle.
The endometrial cycle consist of three phases: proliferation, secretory and menstruation. During proliferation, the lining of the uterus, called the endometrium, expands. In the secretory phase, the epithelial cells of the endometrium secrete glycogen and other material that will support an embryo. If an embryo does not implant into the endometrium, then menstruation or menses commences in which most of the endometrium is sloughed off.
The ovarian cycle contains two phases: follicular and luteal. The follicular phase starts when menses begins and sees several follicles, which contain oocytes, begin the process of development. Release of an oocyte, called ovulation, triggers a change to the luteal phase during which the ovary produces progesterone and estrogen. If an embryo does not implant into the uterus, the ovary will reenter the follicular phase.

Four hormones are the primary drivers of changes during the menstrual cycle: follicle-stimulating hormone (FSH), leutenizing hormone (LH), estrogens and progesterone. The levels of these hormones fluctuate during the menstrual cycle to coordinate the structural and functional changes in the ovary and uterus. The hypothalamus and pituitary are the master controllers of hormone levels during the menstrual cycle. This reading will describe the histological changes in the ovary and uterus throughout the course of the menstrual cycle and the structure and function of the hypothalamus and pituitary that produce the hormones that drive these changes. The reading will also describe the tubes that connect the ovary and uterus and the uterus with the external environment.

Hypothalamus - Pituitary - Gonad Axis
Recall from the reading on the Endocrine System the central role the hypothalamus and anterior pituitary play in controlling the activities of other organs and the production of hormones in these organs. The hypothalamus and anterior pituitary play a similar role in controlling the timing of the menstrual cycle and the structural changes that occur.
The anterior pituitary contains gonadotrophs which are cells that synthesize and release two hormones: follicle-stimulating hormone (FSH) and leutenizing hormone (LH); FSH and LH directly regulate the production of estrogens and progesterone in the ovary.
FSH and LH have a similar structure. Both are heterodimers of an alpha subunit and a beta subunit. FSH and LH share the same alpha subunit but are composed of different beta subunits.
Neurons in the hypothalamus stimulate gonadotrophs to produce and release FSH and LH through the hormone gonadotropin-releasing hormone (GnRH). Gonadotrophs in the anterior pituitary have a receptor for GnRH which stimulates the expression and release of FSH and LH. Hypothalamic neurons release GnRH in a pulsatile fashion (every 30 to 120 minutes), and the rhythmic pattern of GnRH concentration induces gonadotrophs to increase expression of GnRH receptor making them more sensitive to GnRH. Continuous release of GnRH causes gonadotrophs to decrease expression of GnRH receptor.
The frequency of GnRH production can preferentially stimulate production of FSH or LH; higher frequency of GnRH release favors LH production. The frequency of GnRH release increases during the menstrual cycle which in part accounts for the changes in levels of LH and FSH over the course of the menstrual cycle. The hypothalamic neurons that produce GnRH are themselves regulated by other neurons to generate pulsatile releases of GnRH.
The structural relationship between the hypothalamus and pituitary is shown in the slide below. Neurons in the hypothalamus release GnRH which diffuses into a fenestrated capillaries. These capillaries coalesce into portal veins that travel to the anterior pituitary and then ramify into fenestrated capillaries to allow GnRH (and other releasing hormones produced in the hypothalamus) to diffuse out of the circulatory system and into the parenchyma of the anterior pituitary where gonadotrophs and other hormone-secreting cells reside.

Gonadotrophs in the anterior pituitary contain G-protein coupled receptors that bind GnRH. Recall that the cytosolic surface of G-protein coupled receptors interact with heterotrimeric GTP-binding proteins. Upon binding GnRH, the receptors catalyze the alpha-subunit to bind GTP which then triggers activation of several downstream pathways that increase cytosolic calcium and activate both protein kinase A and C. Ultimately, these pathways increase production of FSH and LH and secretion of both hormones.
FSH and LH stimulate cells in the ovary to produce and release estrogens and progesterones. For most of the menstrual cycle estrogens and progesterones generate negative feedback on FSH and LH by inhibiting release of GnRH in the hypothalamus and release of FSH and LH in the anterior pituitary. However, two days before ovulation, estrogens cause gonadotrophs to increase expression GnRH receptor making them more sensitive to GnRH. This leads to a surge of LH (and to lesser extent FSH) production. This LH surge is what triggers ovulation and release of an egg from an ovary.
Prolonged exposure (days to weeks) to high levels of GnRH leads to reduced production of FSH and LH. In most G-protein-coupled receptor signaling pathways, inactivating the receptor or reducing the concentration of the receptor through endocytosis inhibits the activity of downstream signaling pathway. This form of inactivation can be generated in minutes.
The GnRH receptor lacks the cytosolic domain that allows cells to inactivate the receptor. Thus, over short time periods high levels of GnRH do not lead to a decrease in FSH and LH production. Instead, long-term exposure to GnRH eventually inactivate downstream components of the GnRH receptor pathway (e.g. IP3 receptor) which reduces production of FSH and LH. Because this mechanism of inactivate takes longer than inactivating the receptor, it takes several days of exposure to GnRH before FSH and LH levels fall.
Ovary
The human ovary consists of an inner medulla and outer cortex with indistinct boundaries. The medulla contains blood vessels and nerves, while the cortex is occupied by developing follicles. An ovarian follicle progresses through several distinct phases before it is ready to release its ovum into the uterine tube. From the time it starts to develop until it is ready to release its oocyte takes about one year in humans. A cross-section of an ovary reveals follicles in different stages of development as every menstrual cycle several follicles enter the development process.. FSH, LH and various growth factors drive the development of follicles. The next sections will describe the histological features of each stage of follicular development and the major functional changes in the cells compose follicles.

Primordial Follicle
During the first five months of fetal development, a finite number (approximately 2 million) of primordial follicles form in the fetal ovary. A primordial follicle comprises an oocyte surrounded by a single layer of squamous granulosa cells. The oocyte in primordial follicles is arrested in the first meiotic division.

Early Primary Follicle
At the start of each menstrual cycle several primordial follicles start to develop into primary follicles. The first histological change is the granulosa cells which surround the oocyte grow from squamous to cuboidal. The granulosa cells also begin to slowly divide and start to express a receptor for FSH. Because the cells are now sensitive to FSH, they are also called follicular cells. Gap junctions connect the granulosa cells to each other and connect the granulosa cells with the oocyte. Gap junctions allow the diffusion of small molecules between granulosa cells and the oocyte and some of these small molecules keep the oocyte arrested in meiosis.
After the granulosa cells start to develop, the oocyte begins to increase in size. The granulosa cells stimulate oocyte growth by passing nutrients and regulatory factors into the oocyte through gap junctions.

Late Primary Follicle
The late primary follicle stage is reached when the granulosa cells proliferate into a stratified epithelium known as the zona granulosa. In addition, a thin band of glycoproteins that separates the oocyte and follicular cells is seen. This band of glycoproteins is called the zona pellucida. The zone pellucida enlarges as the follicle develops and will accompany the oocyte if it is released from the ovary. Proteins on the surface of sperm will bind to specific glycoproteins in the zona pellucida to aid fertilization.

Just outside the granulosa cells is a layer of cells called theca cells. These cells express a receptor to LH which stimulates the cells to proliferate and synthesize androgens. A thin basement membrane separates the granulosa cells from the theca cells.
Secondary Follicle
The characteristic feature that distinguishes secondary from primary follicles is the appearance of a follicular antrum within the granulosa layer. The antrum contains fluid which is rich in hyaluronan and proteoglycans. Note the increase in cell layers of the zone granulosa, the thicker zone pellucida, and larger oocyte. At this stage, the theca cells have separated into two distinct functional types. Theca interna cells are closest to the granulosa cells and contribute to the production of estrogen. Cells in the theca interna express a receptor for LH. Cells in the theca externa more closely resemble smooth muscle cells.

The production of estrogen requires cells in both the theca interna and zona granulosa. Estrogens, like all steroid hormones, are produced from cholesterol through a multi-step process that requires several different enzymes. Neither the cells of the theca interna nor the granulosa cells contain all of the enzymes necessary to convert cholesterol into estrogens. Theca cells contain enzymes that catalyze the initial conversion of cholesterol into androgens, such as androstenedione, but lack aromatase that carries out the final steps of converting androgens into estrogens. Consequently, androgens produced by theca cells diffuse into the granulosa cells which contain aromatase to convert androgens into estrogen but lack the enzymes for the initial steps in estrogen synthesis. The theca cells are in a better position to catalyze the initial steps in estrogen synthesis because they are closer to blood vessels and can take up LDL to obtain cholesterol.

FSH and LH increase the production of estrogen. LH stimulates cells in the theca interna to increase expression of enzymes that generate androgens, and FSH stimulates cells in the zona granulosa to increase expression of aromatase.
Graafian Follicle
A healthy follicle will continue to grow until it reaches a stage called the Graafian follicle. In a Graaafian follicle, the fluid-filled antrum has increased in volume and pushed the oocyte to one side of the follicle. The oocyte is surrounded by granulosa cells, most of which have detached from the zona granulosa. These granulosa cells are called the corona radiata. Granulosa cells on one side of the oocyte remain attached to the zona granulosa to keep it anchored at one end of the follicle. A thick zona pellucida separates the corona radiata from the oocyte. Outside the zona granulosa are the theca interna and theca externa which are difficult to distinguish at this magnification. An important change in Graafian follicles is the cells in the zona granulosa begin to express receptors for LH.

As Graafian follicles mature, they increase in size from 0.4 mm to 2 cm in diameter, but in each menstrual cycle only one Graafian follicle reaches full size and releases an oocyte. Every menstrual cycle one Graafian follicle starts to rapidly increase in size and will eventually release its oocyte during ovulation. What determines which Graafian follicle is selected to grow and release its oocyte is unclear. However, the ability of one Graafian follicle to accumulate FSH in its astral fluid appears to be important to sustain the growth of the follicle as FSH levels fall prior to ovulation. Just before ovulation the oocyte in the selected Graafian follicle completes the first meiosis (recall that up to this point the oocyte had been arrested in meiosis I). To initiate ovulation, the selected follicle induces the adjacent epithelium of the ovary capsule to separate to allow the oocyte to be released into the oviduct. Upon its release, the oocyte is still surrounded by the zona pellucida and corona radiata both of which facilitate fertilization.
Atretic Follicle
Every menstrual cycle several primordial follicles will began the process of oogenesis by developing into primary follicles. Almost a year later, one of these primary follicles will become the dominant Graafian follicle and its oocyte will be released during ovulation. The other follicles that had began developed at the same time will be lost through a process called atresia. During atresia, the granulosa cells undergo apoptosis and are replaced by fibrosis material while the oocyte degenerates and collapses. Atresia is triggered in secondary follicles and small Graafian follicles during the fall in FSH levels that occurs in follicular phase of the menstrual cycle. Only the secondary follicles and Graafian follicles that have accumulated enough FSH in their antral fluid survive the fall in circulatory FSH levels.

Corpus Luteum
After release of the ovum, the ovary enters the luteal phase during which the cells of the zona granulosa and theca interna form the corpus luteum. Now the cells are called granulosa lutein cells and theca lutein cells. These cells produce progesterone and to a lesser extent estrogen. The center of corpus luteum contains the remains of a blood clot that formed after ovulation..
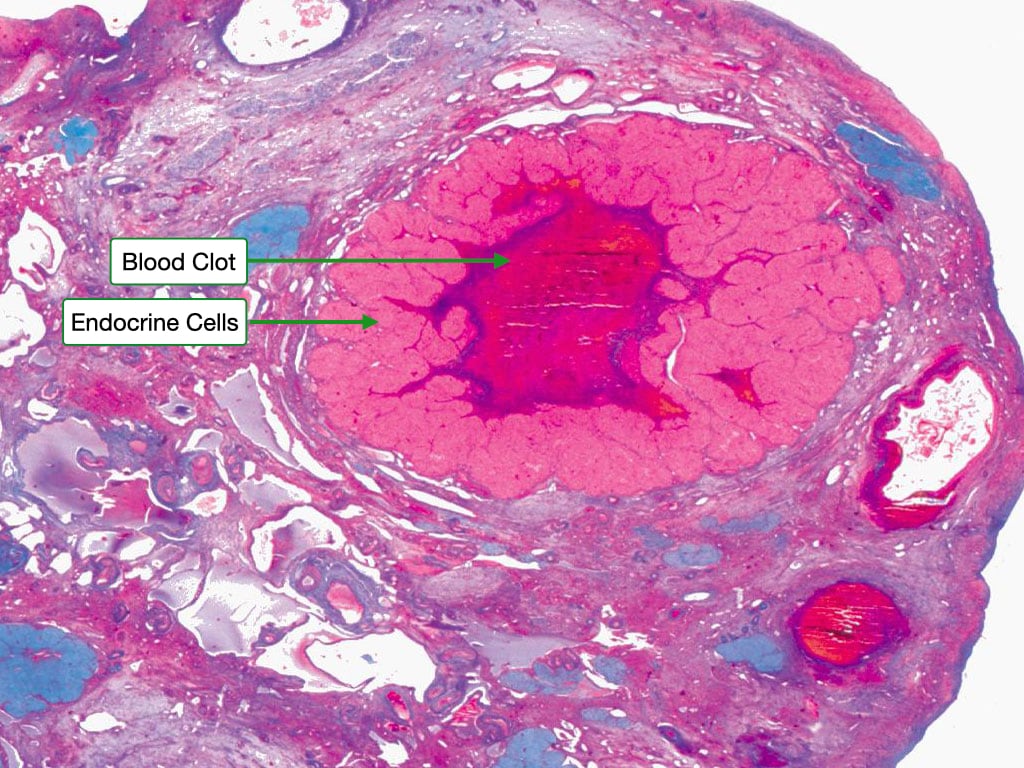
The granulosa lutein cells have an appearance characteristic of steroid-producing cells, with pale cytoplasm indicating the presence of lipid droplets. Theca lutein cells are smaller and more deeply stained. Blood vessels penetrate into region of the granulosa lutein cells allowing them to take up cholesterol to be used to synthesize progesterone.

The activity of the cells of the corpus luteum is sustained by leutenizing hormone. Recall that the cells in the granulosa lutein started to express a receptor to LH in the Graafian follicle. If the ovum is fertilized and implants in the uterine wall, human chorionic gonadotropin replaces leutenizing hormone to sustain the activity of the cells in the corpus luteum.
Corpus Albicans
Cells of the corpus luteum remain active for roughly 14 days and continue to produce progesterone and estrogen under the influence of LH. However, levels of LH fall over the course of the luteal phase and in the absence of fertilization, the corpus luteum will eventually degenerate. The granulosa lutein and theca lutein cells undergo apoptosis and macrophages phagocytose the apoptotic cells. Fibrous material replaces the cells to form a corpus albicans.

If the oocyte is fertilized and successfully implants into the uterus, then cells of the placenta produce human chorionic gonadotropin (hCG) which will sustain the corpus luteum.
Feedback to Control Hormone Levels
Positive and negative feedback play critical roles in regulating the concentrations of hormones during the menstrual cycle. An example of negative feedback involves granulosa cells which are stimulated by FSH to secrete hormones called inhibins. Inhibins act on gonadotrophs to reduce synthesis of beta-subunit of FSH.
Estradiol can generate negative and positive feedback. An example of negative feedback occurs in early stages of the menstrual cycle when estradiol reduces production of GnRH in the hypothalamus. Estradiol generates positive feedback in the mid-cycle phase by increasing the expression of GnRH receptor in gonadotrophs. This makes the cells more sensitive to GnRH and leads in part to the surge of LH that triggers ovulation.
Oviduct
After release from the ovary, the ovum enters the oviduct which is also called the uterine tube. The oviduct consists of several segments: the infundibulum, which lies closest to the ovary, followed by the ampulla, the isthmus, and the pars interstitialis. The first two of these regions have a characteristic appearance that is dominated by an elaborate mucosa that is thrown into numerous branched folds, surrounded by a relatively thin layer of smooth muscle. As the tube moves away from the ovary and toward the uterus, these folds become smaller and the smooth muscle dominates. Coordinated contraction of the smooth muscle layers moves to ovum down the oviduct toward the uterus.

Oviduct Epithelium
The oviduct epithelium consists of two distinct cell types. Ciliated cells dominate and help move the ovum toward the uterus through the wave-like beating of their cilia. Non-ciliated secretory cells, also known as peg cells, release a secretion that lubricates the tube and provides nourishment and protection to the traveling ovum. Note that the epithelium is mostly simple columnar which differs from the pseudostratified, ciliated epithelium in the respiratory tract.

Uterus
The uterus is where an embryo will implant to continue its development. The wall of the uterus is divided into several layers that have distinct structural and functional characteristics. The simplest classification of these layers is their division into a mucosal layer, or endometrium, a muscularis layer, or myometrium, and a serosal layer, or perimetrium. The endometrium itself is divided into two layers, the stratum functionalis and stratum basalis. During the menstrual cycle, the stratum functionalis expands and vascularizes. A fertilized embryo will attach to the epithelium on the surface of the stratum functionalis and then invade into the functionalis. If an embryo does not implant in the functionalis, it is subsequently sloughed off during the process of menstruation. The stratum basalis remains relatively constant and will serve to regenerate the stratum functionalis during the next cycle. The myometrium allows for the expansion and contraction of the uterine cavity and is responsive to the hormone oxytocin.

Uterine Cycle
The endometrium undergoes dramatic structural and functional changes during the menstrual cycle. These changes are divided into two phases: proliferative and secretory.
Proliferative Phase
The proliferative phase is characterized by robust growth of the epithelial cells in the stratum functionalis and the formation of coiled and densely packed glands. These changes in this phase are driven by estrogen.


Secretory Phase
The secretory phase of the uterine cycle begins at ovulation. In this phase, the glands become even more coiled and the functionalis reaches its maximal thickness, whereas the stratum basalis and myometrium remain relatively unchanged. Note the saw-toothed appearance of the glands. Secretions rich in glycogen and glycoprotein can be observed in the lumina of the glands. This phase is drive by progesterone.

Menstrual Phase
If fertilization does not occur, placental tissue does not develop to produce hCG and the corpus luteum in the ovary degenerates. Consequently, the levels of progesterone decrease, causing arteries in the endometrium to constrict and the tissue to become ischemic. This leads to cell death and the sloughing of the stratum functionalis.

Cervix
The cervix lies at the base of the uterus and serves to protect it from bacterial infiltration. It is the site of an important epithelial transition. The upper cervix (endocervix) is lined by a simple columnar epithelium that contains mucous-secreting cells. In contrast, the lower cervix (ectocervix) is lined by a stratified squamous epithelium. The transition point between these two epithelia is known as the external os. Note how the underlying layers of the cervix are composed primarily of collagenous and elastic connective tissue rather than smooth muscle fibers.

Vagina
The vagina is lined by a stratified squamous epithelium that features a small degree of keratinization. Below the epithelium is a thick layer of dense connective tissue, like that in the dermis of the skin. The cells of the vaginal wall typically contain a relatively large amount of cytoplasm because they store and release glycogen. Glycogen is metabolized by commensal bacteria to lactic acid that prevents growth of pathogenic microorganisms.

Male Reproductive System
The male reproductive system consists of the testis which are the sites of sperm generation and the male reproductive tract which deliver sperm from the testis to the outside world. Along the reproductive tract, several secretory organs deliver nutrients and other factors that facilitate survival of sperm.
Hormonal Regulation of Spermatogenesis
Similar to oocyte development in ovaries, development of sperm depends upon the actions of several different hormones, including follicle-stimulating hormone (FSH), leutenizing-hormone (LH), and testosterone. Recall from above the role the hypothalamus and anterior pituitary play in producing FSH and LH. The production of testosterone is described below.
Testis
The testes are a source of sperm and steroid sex hormones. Each testis is a compound tubular gland contained within a thick connective tissue coat called the tunica albuginea. Thin septa radiate from the dorsal portion of the tunica albuginea to separate the testis into lobules. Each lobule contains between one and four seminiferous tubules that are the site of sperm production. Sperm produced in the seminiferous tubules collect in the rete testis before exiting the testis through the ductuli efferentes and entering the male reproductive tract.

This histological image shows the location of the seminiferous tubules within the testis and the tunica albuginea that surrounds the testis. The epididymis is a coiled tube outside the testis where sperm gain motility.

Sperm Development
The development of a mature sperm is divided into two steps, spermatogenesis and spermiogenesis. Spermatogenesis is the process by which an undifferentiated spermatogonium, the stem cell of the testis, develops into a spermatid. During spermatogenesis, the number of chromosomes is halved through meiosis.
Spermiogenesis is the process by which a spermatid matures into a spermatozoan. This process involves the following cellular changes to spermatids.
- An acrosome, containing hydrolytic enzymes, develops and comes to overlie the dense, elongated nucleus.
- A flagellum grows out of the pole opposite the acrosome, facing the tubular lumen. This flagellum is a modified cilium that develops from the centrioles of the spermatid.
- Mitochondria become arranged around the flagellum.
- The bulk of the cytoplasm is cast off as a residual body , leaving only a thin rim of cytoplasm around the nucleus. Sertoli cells consume the residual body.
Spermatogenesis
Seminiferous tubules are coiled tubes that support the development of sperm. This image shows the cross-sections of a tubule. The tubules are lined by a germinal epithelium where sperm develop from stem cells. Similar to other epithelia, the germinal epithelium has basal and apical surfaces. The apical surface faces the lumen of the tube whereas the basal surface interacts with the basement membrane. Just beneath the basement membrane are myoid cells which are contractile and help propel non-motile sperm through the seminiferous tubule.
Situated between seminiferous tubules are collections of cells called Leydig cells. These cells synthesize testosterone.

The germinal epithelia is divided into two compartments: basal and adluminal. The basal compartment is closest to the basement membrane whereas the adluminal compartment is closer to the lumen of the seminiferous tubule. The border between the two compartments is defined by Sertoli cells which are described below.
Spermatogonia rest on the basement membrane of the seminiferous tubule and divide mitotically to produce more spermatogonia and primary spermatocytes. Primary spermatocytes represent the first differentiation step along the spermatogenesis pathway. Spermatogonia remain in the basal compartment while spermatocytes migrate away from the basement membrane and cross into the adluminal compartment toward the lumen of the seminiferous tubule.
Primary spermatocytes enter meiosis and have a prolonged prophase that facilitates the exchange of genetic material between homologous chromosomes. The first meiotic division gives rise to secondary spermatocytes that have 23 pairs of chromatids. This stage is short-lived and consequently, secondary spermatocytes are rarely seen in histological images. This stage ends with the second meiotic division.
The second meiotic division produces spermatids that are haploid. Despite being the product of meiosis, spermatids remain connected to one another by cytoplasmic bridges. These bridges result from incomplete cytokinesis and allow exchange of material for synchronous maturation.
Spermatids go through the structural changes of spermiogenesis, described above, to generate spermatozoa.

Sertoli Cells
Sertoli cells are located within the germinal epithelium and play a supportive role in the development of spermatozoa. These cells have abundant cytoplasm and extend from the basement membrane to the lumen of seminiferous tubules. Sertoli cells have a characteristic oval nucleus with a dark nucleolus.

Sertoli cells facilitate spermatogenesis by providing structural and chemical support to the developing spermatogonia, spermatocytes, spermatids and spermatozoa. In addition, Sertoli cells synthesize androgen-binding protein that keeps testosterone levels high within the seminiferous tubules. Follicle-stimulating hormone (FSH) produced in the anterior pituitary stimulates Sertoli cells to synthesize androgen-binding protein. Sertoli cells also produce inhibin that decreases production of FSH in the anterior pituitary. Thus, Sertoli cells are part of a negative feedback loop that keeps the concentration of FSH within a defined range.
Blood-Testis Barrier
This electron micrograph provides a better view of the structure of Sertoli cells and the blood-testis barrier. The basement membrane on which all Sertoli cells rest is visible, as is a narrow myoid cell which contracts rhythmically. The Sertoli cells are connected to one another via junctional complexes (both tight and adhesion junctions) close to the basement membrane; these complexes divide the germinal epithelium into basal and adluminal compartments. The basal compartment resides between the basement membrane and junctional complexes whereas the adluminal compartment is defined as the region from the junctional complexes to lumen of the seminiferous tubules. The basal compartment contains diploid spermatogonia that rest upon the basement membrane. These cells develop by migrating into the adluminal compartment, which contains primary spermatocytes, spermatids, and spermatozoa.
The primary function of the blood-testis barrier is to create a protected environment, the adluminal compartment, for the development of sperm. The junctional complexes of Sertoli cells prevent the diffusion of antibodies that might bind the surface of sperm and inhibit their motility or ability to fertilize an egg. The junctional complexes also inhibit the diffusion of small molecules that may disrupt the development of sperm or be toxic to sperm.

Leydig Cells
Interstitial or Leydig cells are located in the connective tissue surrounding the seminiferous tubules. They produce testosterone, the male sex hormone responsible for the growth and maintenance of the cells of the germinal epithelium and the development of secondary sex characteristics. Leydig cells express a receptor for LH. When bound to LH, the receptor activates a signaling pathway that increases the synthesis of testosterone. Leydig cells often display cytoplasmic crystals of Reinke, but the function of these crystals is unknown.

Rete Testis
The rete testis connects the seminiferous tubules to the ductus efferentes and the rest of the male reproductive tract. It is lined by ciliated, cuboidal epithelial cells that also contain microvilli. The activity of the cilia helps to move the spermatozoa along the tube, as spermatozoa are immobile until they reach the epididymis. The microvilli absorb excess materials, including protein and potassium, from the seminal fluid.

Male Reproductive Tract
The male reproductive tract is a long tube that brings the spermatozoa from the testes to outside of the body. The tract comprises several segments with different structures and functions. As discussed previously, spermatozoa are produced in the seminiferous tubules in the testis. Spermatozoa flow from the seminiferous tubules into the rete testis and then the ductuli efferentes, which mark the transition from testis to the reproductive tract. The ductuli efferentes merge to form the epididymis which is the site where spermatozoa gain motility. The epididymis transitions into the ductus deferens which receives secretions from the seminal gland and prostate. The ductus deferens from each side merge with the urethra.

Ductuli Efferentes
The ductuli efferentes emerge from the dorso-superior margin of each testis. They originate from the rete testis and gradually fuse to form the epididymis. The epithelium of the ductuli efferentes has a characteristic scalloped appearance that results from a lining that contains both cuboidal and columnar epithelial cells. A layer of smooth muscle surrounds the walls. The non-ciliated cells reabsorb testicular fluid, while the ciliated cells propel the immobile sperm to the epididymis, where they gain the ability to swim.

Epididymis
The epididymis is a muscular and highly convoluted tubule that stores spermatozoa and is the site at which they acquire their motility. It is lined by a pseudostratified, columnar epithelium whose cells contain non-motile stereocilia. These stereocilia absorb much of the excess fluid surrounding the spermatozoa. The epithelium of the epididymis also contains mitotic basal cells. In this section, the spermatozoa can be seen in the lumen throughout the epididymis.

Ductus Deferens
The ductus deferens is another muscular tubule that carries sperm downstream from the epididymis. Its wall is thicker than that of the epididymis and contains three muscular layers: inner longitudinal, middle circular, and outer longitudinal. The epithelium of the ductus deferens is similar to that of the epididymis, with pseudostratified, columnar cells bearing stereocilia.

The distal portion of the ductus deferens is called the ampulla and receives secretions from the seminal vesicles. The duct is now referred to as the ejaculatory duct and ducts from each side will merge and join the urethra as it runs through prostate gland.
Urethra
The urethra is lined primarily by stratified or psueodstratified columnar epithelial cells, but its opening displays a stratified squamous epithelium. In the penis, erectile tissue surrounds the urethra and contains numerous blood vessels. During an erection, the arteries dilate to fill the sinuses, which obstruct venous outflow and traps blood.

Seminal Vesicle
Seminal vesicles are glandular sacs that produce a secretion that composes 70% of the seminal fluid and contains fructose, fibrinogen, and prostaglandins. The secretion empties via a short duct into the ampulla of the ductus deferens. The seminal vesicles appear as honeycombed saccules with thin, highly branched folds of mucosa, lined by a pseudostratified columnar epithelium. Observe the coat of smooth muscle surrounding the saccular dilation of the gland. Its contraction expels the accumulated secretion during ejaculation.

Prostate Gland
The prostate is a walnut-sized conglomeration of tubulo-acinar glands that surrounds the initial segment of the urethra. This gland produces a secretory product containing citric acid and proteolytic enzymes that prevent coagulation of semen. The epithelium that lines the glands is usually columnar with numerous flattened basal cells also visible. The lumen of the glands often contain prostatic concretions that accumulate over time. Their significance is unknown but they make a useful marker for identifying the prostate. The glands are surrounded by a stroma that contains connective tissue and smooth muscle.
